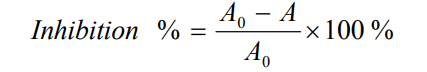Hydroxypropyl methylcellulose (HPMC) is a natural polymer material with abundant resources, renewable, and good water solubility and film-forming properties. It is an ideal raw material for the preparation of water-soluble packaging films.
Water-soluble packaging film is a new type of green packaging material, which has received extensive attention in Europe and the United States and other countries. It is not only safe and convenient to use, but also solves the problem of packaging waste disposal. At present, water-soluble films mainly use petroleum-based materials such as polyvinyl alcohol and polyethylene oxide as raw materials. Petroleum is a non-renewable resource, and large-scale use will cause resource shortages. There are also water-soluble films using natural substances such as starch and protein as raw materials, but these water-soluble films have poor mechanical properties. In this paper, a new type of water-soluble packaging film was prepared by solution casting film-forming method using hydroxypropyl methylcellulose as raw material. The effects of the concentration of HPMC film-forming liquid and film-forming temperature on the tensile strength, elongation at break, light transmittance and water solubility of HPMC water-soluble packaging films were discussed. Glycerol, sorbitol and glutaraldehyde were used Further improve the performance of HPMC water-soluble packaging film. Finally, in order to expand the application of HPMC water-soluble packaging film in food packaging, bamboo leaf antioxidant (AOB) was used to improve the antioxidant properties of HPMC water-soluble packaging film. The main findings are as follows:
(1) With the increase of HPMC concentration, the tensile strength and elongation at break of HPMC films increased, while the light transmittance decreased. When the HPMC concentration is 5% and the film forming temperature is 50°C, the comprehensive properties of the HPMC film are better. At this time, the tensile strength is about 116MPa, the elongation at break is about 31%, the light transmittance is 90%, and the water-dissolving time is 55min.
(2) The plasticizers glycerol and sorbitol improved the mechanical properties of HPMC films, which significantly increased their elongation at break. When the content of glycerol is between 0.05% and 0.25%, the effect is the best, and the elongation at break of HPMC water-soluble packaging film reaches about 50%; when the content of sorbitol is 0.15%, the elongation at break increases to 45% or so. After the HPMC water-soluble packaging film was modified with glycerol and sorbitol, the tensile strength and optical properties decreased, but the decrease was not significant.
(3) Infrared spectroscopy (FTIR) of the glutaraldehyde-crosslinked HPMC water-soluble packaging film showed that glutaraldehyde had cross-linked with the film, reducing the water-solubility of the HPMC water-soluble packaging film. When the addition of glutaraldehyde was 0.25%, the mechanical properties and optical properties of the films reached the optimum. When the addition of glutaraldehyde was 0.44%, the water-dissolving time reached 135 min.
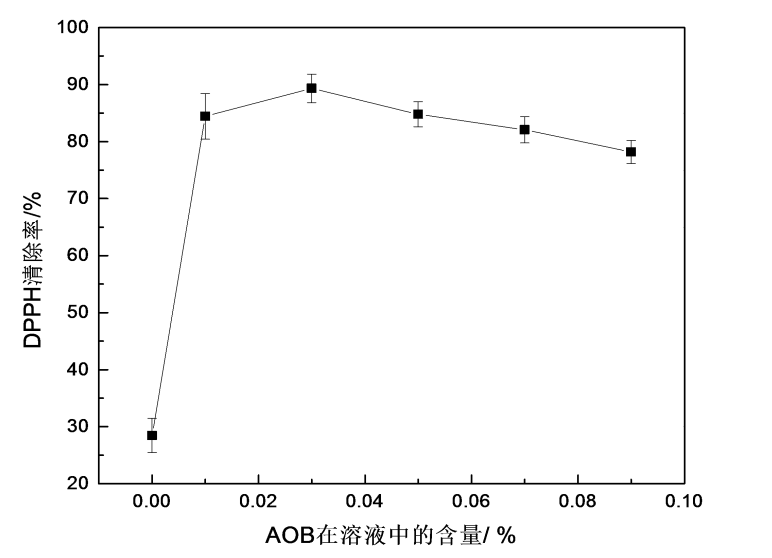
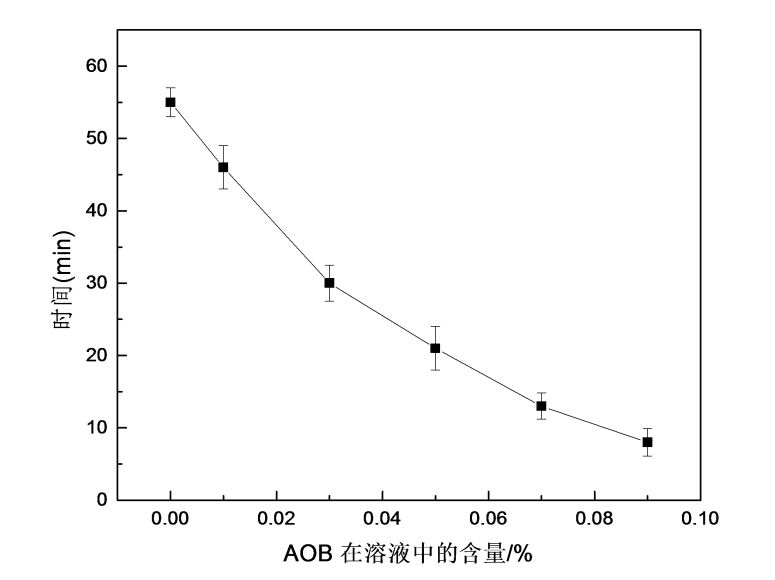
(4) Adding an appropriate amount of AOB to the HPMC water-soluble packaging film film-forming solution can improve the antioxidant properties of the film. When 0.03% AOB was added, the AOB/HPMC film had a scavenging rate of about 89% for DPPH free radicals, and the scavenging efficiency was the best, which was 61% higher than that of the HPMC film without AOB, and the water solubility was also significantly improved.
Key words: water-soluble packaging film; hydroxypropyl methylcellulose; plasticizer; cross-linking agent; antioxidant.
Table of contents
Summary…………………………………………. ……………………………………………… ……………………………………….I
ABSTRACT……………………………………………… ……………………………………………… ……………………………II
Table of contents…………………………………………. ……………………………………………… …………………………i
Chapter One Introduction………………………………………. ……………………………………………… ……………..1
1.1Water- soluble film……………………………………………… ……………………………………………… …………….1
1.1.1Polyvinyl Alcohol (PVA) Water-Soluble Film ………………………………………… ………………… 1
1.1.2Polyethylene oxide (PEO) water-soluble film ………………………………………… …………..2
1.1.3Starch-based water-soluble film………………………………………… ……………………………………….2
1.1.4 Protein-Based Water-Soluble Films………………………………………… ………………………………….2
1.2 Hydroxypropyl methylcellulose …………………………………………….. ………………………………………3
1.2.1 The structure of hydroxypropyl methylcellulose ………………………………………… …………….3
1.2.2 Water solubility of hydroxypropyl methylcellulose ………………………………………… …………4
1.2.3 Film-forming properties of hydroxypropyl methylcellulose ……………………………………….4
1.3 Plasticization modification of hydroxypropyl methylcellulose film………………………………..4
1.4 Cross-linking modification of hydroxypropyl methylcellulose film……………………………….5
1.5 Antioxidative properties of hydroxypropyl methylcellulose film…………………………………. 5
1.6 Proposal of the topic……………………………………………………………. ………………………………………….7
1.7 Research content ………………………………………… ……………………………………………… ………………..7
Chapter 2 Preparation and Properties of Hydroxypropyl Methyl Cellulose Water-Soluble Packaging Film………………………………………………………………………………………………………………………………….8
2.1 Introduction ………………………………………… ……………………………………………… …………………………. 8
2.2 Experimental Section ……………………………………………………………. ………………………………………….8
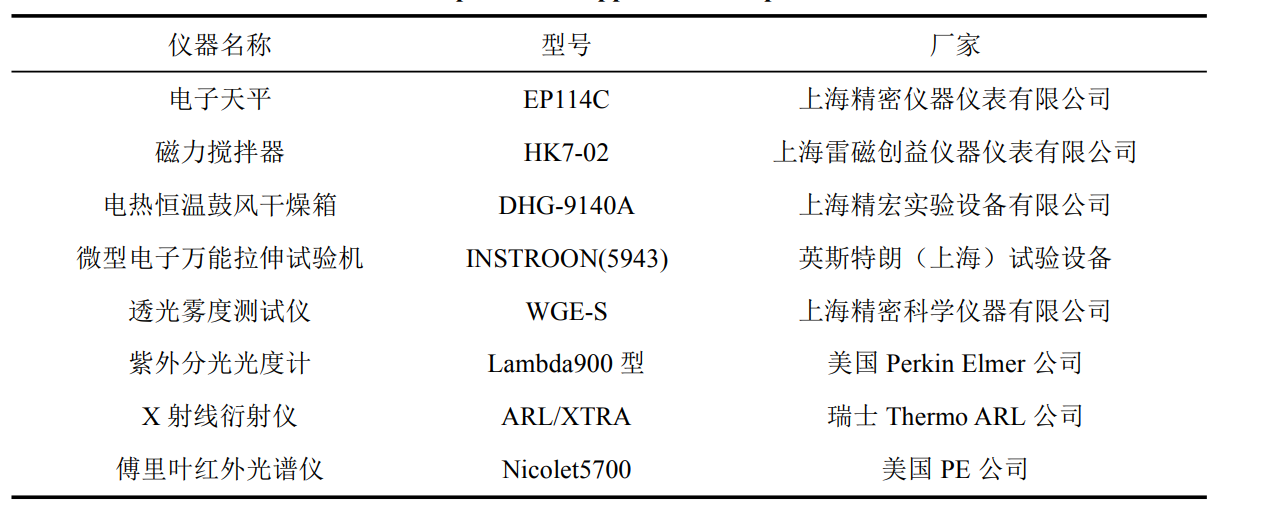
2.2.1 Experimental materials and instruments……………………………………………………………. ………..8
2.2.2 Specimen Preparation ………………………………………… ………………………………………………………..9
2.2.3 Characterization and performance testing ……………………………………….. ……………………….9
2.2.4 Data processing…………………………………………. ……………………………………………… ………………10
2.3 Results and Discussion ………………………………………… ……………………………………………… ………10
2.3.1 The effect of film-forming solution concentration on HPMC thin films ………………………….. …………………………………………………………………………………………………………………. 10
2.3.2 Influence of film formation temperature on HPMC thin films ………………………………………… ……………………………………………………………………………………………………..13
2.4 Chapter Summary ………………………………………… ……………………………………….. 16
Chapter 3 Effects of Plasticizers on HPMC Water-Soluble Packaging Films ……………………………………………………………………..17
3.1 Introduction …………………………………………………………… ……………………………………………… 17
3.2 Experimental Section ……………………………………………… ……………………………………………… ………..17
3.2.1 Experimental materials and instruments ………………………………………… ……………………………17
3.2.2 Specimen Preparation ………………………………………… ……………………………18
3.2.3 Characterization and performance testing ……………………………………….. …………………….18
3.2.4 Data processing………………………………………………………. ………………………………………..19
3.3 Results and Discussion ………………………………………… …………………………………………19
3.3.1 The effect of glycerol and sorbitol on the infrared absorption spectrum of HPMC thin films …………………………………………………………………………………………………………………………….19
3.3.2 The effect of glycerol and sorbitol on the XRD patterns of HPMC thin films ……………………………………………………………………………………………………………………………………..20
3.3.3 Effects of glycerol and sorbitol on the mechanical properties of HPMC thin films……………………………………………………………………………………………………………………………………….21
3.3.4 Effects of glycerol and sorbitol on the optical properties of HPMC films………………………………………………………………………………………………………………………………………22
3.3.5 The influence of glycerol and sorbitol on the water solubility of HPMC films………. 23
3.4 Chapter Summary ………………………………………… ……………………………………………………..24
Chapter 4 Effects of Crosslinking Agents on HPMC Water-Soluble Packaging Films ……………………………………………………………………………………………………………………………………25
4.1 Introduction …………………………………………………………… …………………………………………. 25
4.2 Experimental Section ……………………………………………… …………………………………………25
4.2.1 Experimental materials and instruments ………………………………………… ……………25
4.2.2 Specimen Preparation ………………………………………… ………………………………………..26
4.2.3 Characterization and performance testing ……………………………………….. ………….26
4.2.4 Data processing……………………………………………………………. ………………………………………..26
4.3 Results and Discussion …………………………………………………………… …………………………………27
4.3.1 Infrared absorption spectrum of glutaraldehyde-crosslinked HPMC thin films……………………………………………………………………………………………………………………………………………..27
4.3.2 XRD patterns of glutaraldehyde cross-linked HPMC thin films…………………………..27
4.3.3 The effect of glutaraldehyde on the water solubility of HPMC films…………………..28
4.3.4 The effect of glutaraldehyde on the mechanical properties of HPMC thin films … 29
4.3.5 The effect of glutaraldehyde on the optical properties of HPMC films …………………29
4.4 Chapter Summary ………………………………………… ……………………………………….. 30
Chapter 5 Natural Antioxidant HPMC Water-Soluble Packaging Film …………………………..31
5.1 Introduction …………………………………………………………… ………………………………………………………31
5.2 Experimental Section ……………………………………………… ………………………………………………………31
5.2.1 Experimental materials and experimental instruments………………………………………………31
5.2.2 Specimen Preparation ………………………………………… …………………………………………………….32
5.2.3 Characterization and performance testing ……………………………………….. ………………………32
5.2.4 Data processing………………………………………………………. ……………………………………………………33
5.3 Results and Analysis ………………………………………… ……………………………………………… …………….33
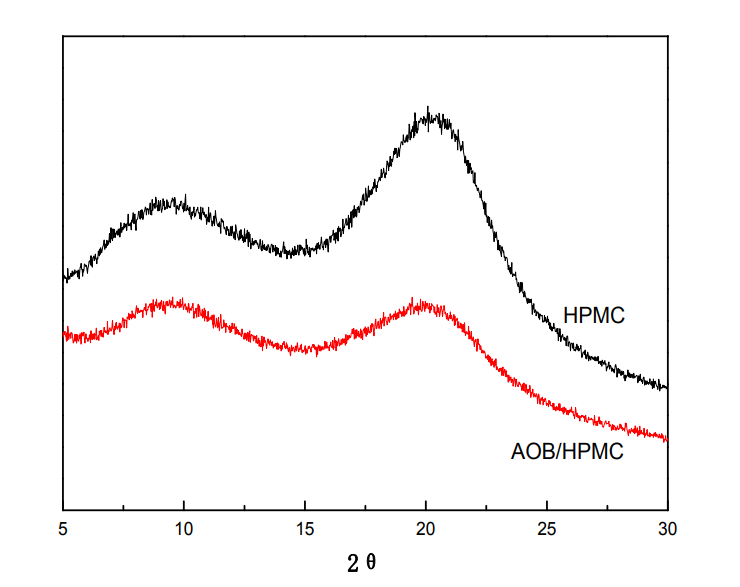
5.3.1 FT-IR analysis ………………………………………… ……………………………………………… ………… 33
5.3.2 XRD analysis ………………………………………… ……………………………………………… ………..34
5.3.3 Antioxidant properties ………………………………………… ……………………………………………… 34
5.3.4 Water solubility ………………………………………… ……………………………………………… …………….35
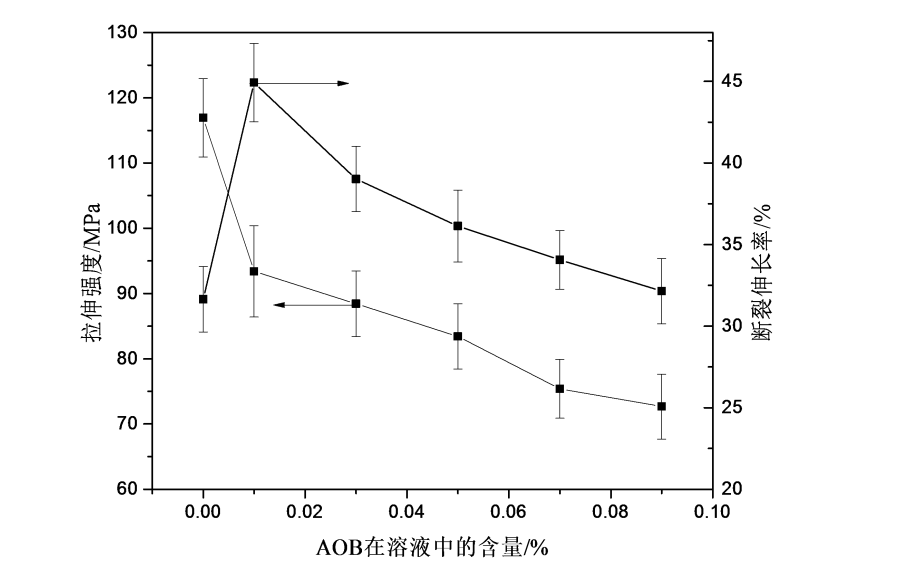
5.3.5 Mechanical properties ………………………………………… ………………………………………………..36
5.3.6 Optical performance ……………………………………………… …………………………………………37
5.4 Chapter Summary ………………………………………… ……………………………………………… ……….37
Chapter 6 Conclusion ……………………………………………………………. ……………………………………..39
references………………………………………… ……………………………………………… …………………………… 40
Research outputs during degree studies ………………………………………… …………………………..44
Acknowledgments ………………………………………… ……………………………………………… ……………….46
Chapter One Introduction
As a novel green packaging material, water-soluble packaging film has been widely used in the packaging of various products in foreign countries (such as the United States, Japan, France, etc.) [1]. Water-soluble film, as the name implies, is a plastic film that can be dissolved in water. It is made of water-soluble polymer materials that can dissolve in water and is prepared by a specific film-forming process. Due to its special properties, it is very suitable for people to pack. Therefore, more and more researchers have begun to pay attention to the requirements of environmental protection and convenience [2].
1.1 Water-soluble film
At present, water-soluble films are mainly water-soluble films using petroleum-based materials such as polyvinyl alcohol and polyethylene oxide as raw materials, and water-soluble films using natural substances such as starch and protein as raw materials.
1.1.1 Polyvinyl Alcohol (PVA) Water-Soluble Film
At present, the most widely used water-soluble films in the world are mainly water-soluble PVA films. PVA is a vinyl polymer that can be used by bacteria as a carbon source and energy source, and can be decomposed under the action of bacteria and enzymes [3] ], which belongs to a kind of biodegradable polymer material with low price, excellent oil resistance, solvent resistance and gas barrier properties [4]. PVA film has good mechanical properties, strong adaptability and good environmental protection. It has been widely used and has a high degree of commercialization. It is by far the most widely used and the largest water-soluble packaging film in the market [5]. PVA has good degradability and can be decomposed by microorganisms to generate CO2 and H2O in the soil [6]. Most of the research on water-soluble films now is to modify and blend them to obtain better water-soluble films. Zhao Linlin, Xiong Hanguo [7] studied the preparation of a water-soluble packaging film with PVA as the main raw material, and determined the optimal mass ratio by orthogonal experiment: oxidized starch (O-ST) 20%, gelatin 5% , glycerol 16%, sodium dodecyl sulfate (SDS) 4%. After microwave drying of the obtained film, the water-soluble time in water at room temperature is 101s.
Judging from the current research situation, PVA film is widely used, low cost, and excellent in various properties. It is the most perfect water-soluble packaging material at present. However, as a petroleum-based material, PVA is a non-renewable resource, and its raw material production process may be polluted. Although the United States, Japan and other countries have listed it as a non-toxic substance, its safety is still open to question. Both inhalation and ingestion are harmful to the body [8], and it cannot be called a complete green chemistry.
1.1.2 Polyethylene oxide (PEO) water-soluble film
Polyethylene oxide, also known as polyethylene oxide, is a thermoplastic, water-soluble polymer that can be mixed with water in any ratio at room temperature [9]. The structural formula of polyethylene oxide is H-(-OCH2CH2-) n-OH, and its relative molecular mass will affect its structure. When the molecular weight is in the range of 200~20000, it is called polyethylene glycol (PEG), and the molecular weight is greater than 20,000 can be called polyethylene oxide (PEO) [10]. PEO is a white flowable granular powder, which is easy to process and shape. PEO films are usually prepared by adding plasticizers, stabilizers and fillers to PEO resins through thermoplastic processing [11].
PEO film is a water-soluble film with good water solubility at present, and its mechanical properties are also good, but PEO has relatively stable properties, relatively difficult degradation conditions, and slow degradation process, which has a certain impact on the environment, and most of its main functions can be used. PVA film alternative [12]. In addition, PEO also has certain toxicity, so it is rarely used in product packaging [13].
1.1.3 Starch-based water-soluble film
Starch is a natural high molecular polymer, and its molecules contain a large number of hydroxyl groups, so there is a strong interaction between starch molecules, so that starch is difficult to melt and process, and the compatibility of starch is poor, and it is difficult to interact with other polymers. processed together [14,15]. The water solubility of starch is poor, and it takes a long time to swell in cold water, so modified starch, that is, water-soluble starch, is often used to prepare water-soluble films. Generally, starch is chemically modified by methods such as esterification, etherification, grafting, and cross-linking to change the original structure of starch, thereby improving the water-solubility of starch [7,16].
Introduce ether bonds into starch groups by chemical means or use strong oxidants to destroy the inherent molecular structure of starch to obtain modified starch with better performance [17], and to obtain water-soluble starch with better film-forming properties. However, at low temperature, starch film has extremely poor mechanical properties and poor transparency, so in most cases, it needs to be prepared by blending with other materials such as PVA, and the actual use value is not high.
1.1.4 Protein-based water-soluble thin
Protein is a biologically active natural macromolecular substance contained in animals and plants. Since most protein substances are insoluble in water at room temperature, it is necessary to solve the solubility of proteins in water at room temperature to prepare water-soluble films with proteins as materials. In order to improve the solubility of proteins, they need to be modified. Common chemical modification methods include dephthalemination, phthaloamidation, phosphorylation, etc. [18]; the effect of modification is to change the tissue structure of the protein, thereby increasing the solubility, gelation, Functionalities such as water absorption and stability meet the needs of production and processing. Protein-based water-soluble films can be produced by using agricultural and sideline product wastes such as animal hairiness as raw materials, or by specializing in the production of high-protein plants to obtain raw materials, without the need for petrochemical industry, and the materials are renewable and have less impact on the environment [19]. However, the water-soluble films prepared by the same protein as the matrix have poor mechanical properties and low water solubility at low temperature or room temperature, so their application range is narrow.
To sum up, it is of great significance to develop a new, renewable, water-soluble packaging film material with excellent performance to improve the deficiencies of current water-soluble films.
Hydroxypropyl Methyl Cellulose (HydroxyPropyl Methyl Cellulose, HPMC for short) is a natural polymer material, not only rich in resources, but also non-toxic, harmless, low-cost, not competing with people for food, and an abundant renewable resource in nature [20] ]. It has good water solubility and film-forming properties, and has the conditions for preparing water-soluble packaging films.
1.2 Hydroxypropyl methylcellulose
Hydroxypropyl Methyl Cellulose (HydroxyPropyl Methyl Cellulose, HPMC for short), also abbreviated as hypromellose, is obtained from natural cellulose through alkalization treatment, etherification modification, neutralization reaction and washing and drying processes. A water-soluble cellulose derivative [21]. Hydroxypropyl methylcellulose has the following characteristics:
(1) Abundant and renewable sources. The raw material of hydroxypropyl methylcellulose is the most abundant natural cellulose on earth, which belongs to organic renewable resources.
(2) Environmentally friendly and biodegradable. Hydroxypropyl methylcellulose is non-toxic and harmless to human body and can be used in medicine and food industries.
(3) Wide range of uses. As a water-soluble polymer material, hydroxypropyl methylcellulose has good water solubility, dispersion, thickening, water retention and film-forming properties, and can be widely used in building materials, textiles, etc. , food, daily chemicals, coatings and electronics and other industrial fields [21].
1.2.1 Structure of hydroxypropyl methylcellulose
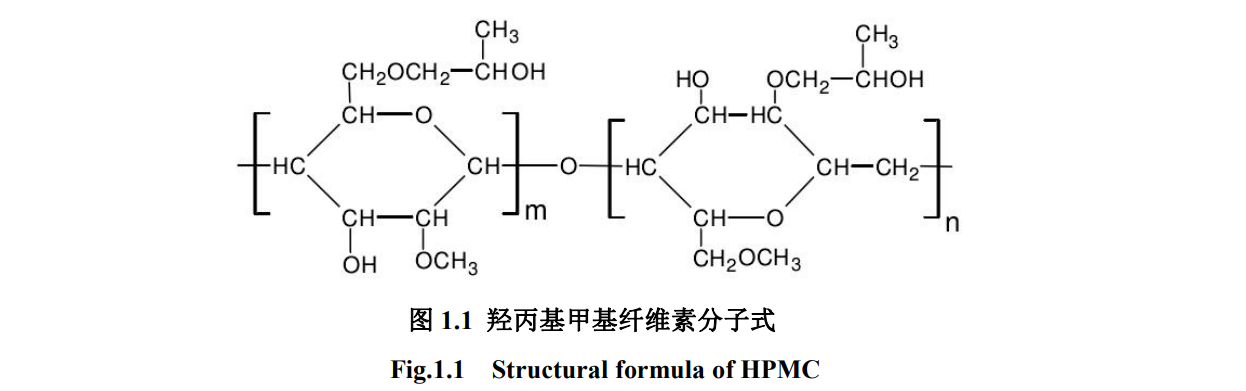
HPMC is obtained from natural cellulose after alkalization, and part of its polyhydroxypropyl ether and methyl are etherified with propylene oxide and methyl chloride. The general commercialized HPMC methyl substitution degree ranges from 1.0 to 2.0, and the hydroxypropyl average substitution degree ranges from 0.1 to 1.0. Its molecular formula is shown in Figure 1.1 [22]
Due to the strong hydrogen bonding between natural cellulose macromolecules, it is difficult to dissolve in water. The solubility of etherified cellulose in water is significantly improved because ether groups are introduced into etherified cellulose, which destroys the hydrogen bonds between cellulose molecules and increases its solubility in water [23] ]. Hydroxypropyl methylcellulose (HPMC) is a typical hydroxyalkyl alkyl mixed ether [21], its structural unit D-glucopyranose residue contains methoxy (-OCH3), hydroxypropoxy (-OCH2 CH-(CH3 ) n OH) and unreacted hydroxyl groups, the performance of cellulose mixed ethers is a comprehensive reflection of the coordination and contribution of each group. -[OCH2CH(CH3)] n OH The hydroxyl group at the end of the n OH group is an active group, which can be further alkylated and hydroxyalkylated, and the branched chain is longer, which has a certain internal plasticizing effect on the macromolecular chain; -OCH3 is a end-capping group, the reaction site will be inactivated after substitution, and it belongs to a short-structured hydrophobic group [21]. The hydroxyl groups on the newly added branch chain and the hydroxyl groups remaining on the glucose residues can be modified by the above groups, resulting in extremely complex structures and adjustable properties within a certain energy range [24].
1.2.2 Water solubility of hydroxypropyl methylcellulose
Hydroxypropyl methylcellulose has many excellent properties due to its unique structure, the most notable of which is its water solubility. It swells into a colloidal solution in cold water, and the solution has certain surface activity, high transparency and stable performance [21]. Hydroxypropyl methylcellulose is actually a cellulose ether obtained after methylcellulose is modified by propylene oxide etherification, so it still has the characteristics of cold-water solubility and hot water insolubility similar to methylcellulose [ 21], and its water solubility in water was improved. Methyl cellulose needs to be placed at 0 to 5 °C for 20 to 40 minutes to obtain a product solution with good transparency and stable viscosity [25]. The solution of hydroxypropyl methylcellulose product only needs to be at 20-25 °C to achieve good stability and good transparency [25]. For example, the pulverized hydroxypropyl methylcellulose (granular shape 0.2-0.5 mm) can be easily dissolved in water at room temperature without cooling when the viscosity of 4% aqueous solution reaches 2000 centipoise at 20°C.
1.2.3 Film-forming properties of hydroxypropyl methylcellulose
Hydroxypropyl methylcellulose solution has excellent film-forming properties, which can provide good conditions for the coating of pharmaceutical preparations. The coating film formed by it is colorless, odorless, tough and transparent [21].
Yan Yanzhong [26] used an orthogonal test to investigate the film-forming properties of hydroxypropyl methylcellulose. Screening was carried out at three levels with different concentrations and different solvents as factors. The results showed that adding 10% hydroxypropyl methylcellulose into 50% ethanol solution had the best film-forming properties, and could be used as a film-forming material for sustained-release drug films.
1.1 Plasticization modification of hydroxypropyl methylcellulose film
As a natural renewable resource, the film prepared from cellulose as a raw material has good stability and processability, and is biodegradable after being discarded, which is harmless to the environment. However, unplasticized cellulose films have poor toughness, and cellulose can be plasticized and modified.
[27] used triethyl citrate and acetyl tetrabutyl citrate to plasticize and modify cellulose acetate propionate. The results showed that the elongation at break of the cellulose acetate propionate film was increased by 36% and 50% when the mass fraction of triethyl citrate and acetyl tetrabutyl citrate was 10%.
Luo Qiushui et al [28] studied the effects of plasticizers glycerol, stearic acid and glucose on the mechanical properties of methylcellulose membranes. The results showed that the elongation rate of methyl cellulose membrane was better when the glycerol content was 1.5%, and the elongation ratio of methyl cellulose membrane was better when the addition content of glucose and stearic acid was 0.5%.
Glycerol is a colorless, sweet, clear, viscous liquid with a warm sweet taste, commonly known as glycerin. Suitable for analysis of aqueous solutions, softeners, plasticizers, etc. It can be dissolved with water in any proportion, and the low-concentration glycerol solution can be used as lubricating oil to moisturize the skin. Sorbitol, white hygroscopic powder or crystalline powder, flakes or granules, odorless. It has the functions of moisture absorption and water retention. Adding a little in the production of chewing gum and candy can keep the food soft, improve the organization and reduce the hardening and play the role of sand. Glycerol and sorbitol are both water-soluble substances, which can be mixed with water-soluble cellulose ethers [23]. They can be used as plasticizers for cellulose. After adding, they can improve the flexibility and elongation at break of cellulose films. [29]. Generally, the concentration of the solution is 2-5%, and the amount of plasticizer is 10-20% of cellulose ether. If the content of plasticizer is too high, the shrinkage phenomenon of colloid dehydration will occur at high temperature [30].
1.2 Crosslinking modification of hydroxypropyl methylcellulose film
The water-soluble film has good water solubility, but it is not expected to dissolve quickly when used in some occasions, such as seed packaging bags. The seeds are wrapped with a water-soluble film, which can increase the survival rate of the seeds. At this time, in order to protect the seeds, it is not expected that the film will dissolve quickly, but the film should first play a certain water-retaining effect on the seeds. Therefore, it is necessary to prolong the water-soluble time of the film. [21].
The reason why hydroxypropyl methylcellulose has good water solubility is that there are a large number of hydroxyl groups in its molecular structure, and these hydroxyl groups can undergo cross-linking reaction with aldehydes to make hydroxypropyl methylcellulose molecules The hydroxyl hydrophilic groups of hydroxypropyl methylcellulose are reduced, thereby reducing the water solubility of the hydroxypropyl methylcellulose film, and the cross-linking reaction between hydroxyl groups and aldehydes will generate many chemical bonds, which can also improve the mechanical properties of the film to a certain extent. The aldehydes cross-linked with hydroxypropyl methylcellulose include glutaraldehyde, glyoxal, formaldehyde, etc. Among them, glutaraldehyde has two aldehyde groups, and the cross-linking reaction is fast, and glutaraldehyde is a commonly used disinfectant. It is relatively safe, so glutaraldehyde is generally used as the cross-linking agent for ethers. The amount of this type of cross-linking agent in the solution is generally 7 to 10% of the weight of the ether. The treatment temperature is about 0 to 30 ° C, and the time is 1 ~120 minutes [31]. The cross-linking reaction needs to be carried out under acidic conditions. First, an inorganic strong acid or organic carboxylic acid is added to the solution to adjust the pH of the solution to about 4-6, and then aldehydes are added to carry out the cross-linking reaction [32]. Acids used include HCl, H2SO4, acetic acid, citric acid, and the like. The acid and aldehyde can also be added at the same time to make the solution carry out the cross-linking reaction in the desired pH range [33].
1.3 Antioxidative properties of hydroxypropyl methylcellulose films
Hydroxypropyl methylcellulose is rich in resources, easy to form film, and has good fresh-keeping effect. As a food preservative, it has great development potential [34-36].
Zhuang Rongyu[37] used hydroxypropyl methylcellulose (HPMC) edible film, coated it on tomato, and then stored it at 20 °C for 18 days to study its effect on tomato firmness and color. The results show that the hardness of tomato with HPMC coating is higher than that without coating. It was also proved that HPMC edible film could delay the color change of tomatoes from pink to red when stored at 20℃.
[38] studied the effects of hydroxypropyl methylcellulose (HPMC) coating treatment on the quality, anthocyanin synthesis and antioxidant activity of “Wuzhong” bayberry fruit during cold storage. The results showed that the anti-oxidation performance of bayberry treated with HPMC film was improved, and the decay rate during storage was decreased, and the effect of 5% HPMC film was the best.
Wang Kaikai et al. [39] used “Wuzhong” bayberry fruit as the test material to study the effect of riboflavin-complexed hydroxypropyl methylcellulose (HPMC) coating on the quality and antioxidant properties of postharvest bayberry fruit during storage at 1 ℃. effect of activity. The results showed that the riboflavin-composite HPMC-coated bayberry fruit was more effective than the single riboflavin or HPMC coating, effectively reducing the decay rate of bayberry fruit during storage, thereby prolonging the storage period of the fruit.
In recent years, people have higher and higher requirements for food safety. Researchers at home and abroad have gradually shifted their research focus from food additives to packaging materials. By adding or spraying antioxidants into packaging materials, they can reduce food oxidation. The effect of decay rate [40]. Natural antioxidants have been widely concerned because of their high safety and good health effects on the human body [40,41].
Antioxidant of Bamboo Leaves (AOB for short) is a natural antioxidant with unique natural bamboo fragrance and good water solubility. It has been listed in the national standard GB2760 and has been approved by the Ministry of Health as an antioxidant for natural food. It can also be used as a food additive for meat products, aquatic products and puffed food [42].
Sun Lina etc.[42] reviewed the main components and properties of bamboo leaf antioxidants and introduced the application of bamboo leaf antioxidants in food. Adding 0.03% AOB to fresh mayonnaise, the antioxidant effect is the most obvious at this time. Compared with the same amount of tea polyphenol antioxidants, its antioxidant effect is obviously better than that of tea polyphenols; adding 150% to beer At mg/L, the antioxidant properties and storage stability of beer are significantly increased, and the beer has good compatibility with the wine body. While ensuring the original quality of the wine body, it also increases the aroma and mellow taste of bamboo leaves [43].
In summary, hydroxypropyl methylcellulose has good film-forming properties and excellent performance. It is also a green and degradable material, which can be used as a packaging film in the field of packaging [44-48]. Glycerol and sorbitol are both water-soluble plasticizers. Adding glycerol or sorbitol to the cellulose film-forming solution can improve the toughness of the hydroxypropyl methylcellulose film, thereby increasing the elongation at break of the film [ 49-51]. Glutaraldehyde is a commonly used disinfectant. Compared with other aldehydes, it is relatively safe, and has a dialdehyde group in the molecule, and the cross-linking speed is relatively fast. It can be used as a cross-linking modification of hydroxypropyl methylcellulose film. It can adjust the water solubility of the film, so that the film can be used in more occasions [52-55]. Adding bamboo leaf antioxidants to hydroxypropyl methylcellulose film to improve the antioxidant properties of hydroxypropyl methylcellulose film and expand its application in food packaging.
1.4 Proposal of the topic
From the current research situation, water-soluble films are mainly composed of PVA films, PEO films, starch-based and protein-based water-soluble films. As a petroleum-based material, PVA and PEO are non-renewable resources, and the production process of their raw materials may be polluted. Although the United States, Japan and other countries have listed it as a non-toxic substance, its safety is still open to question. Both inhalation and ingestion are harmful to the body [8], and it cannot be called a complete green chemistry. The production process of starch-based and protein-based water-soluble materials is basically harmless and the product is safe, but they have the disadvantages of hard film formation, low elongation, and easy breakage. Therefore, in most cases, they need to be prepared by blending with other materials such as PVA. The use value is not high. Therefore, it is of great significance to develop a new, renewable, water-soluble packaging film material with excellent performance to improve the defects of the current water-soluble film.
Hydroxypropyl methylcellulose is a natural polymer material, which is not only rich in resources, but also renewable. It has good water solubility and film-forming properties, and has the conditions for preparing water-soluble packaging films. Therefore, this paper intends to prepare a new type of water-soluble packaging film with hydroxypropyl methylcellulose as raw material, and systematically optimize its preparation conditions and ratio, and add appropriate plasticizers (glycerol and sorbitol). ), cross-linking agent (glutaraldehyde), antioxidant (bamboo leaf antioxidant), and improve their properties, in order to prepare hydroxypropyl group with better comprehensive properties such as mechanical properties, optical properties, water solubility and antioxidant properties. Methylcellulose water-soluble packaging film is of great significance for its application as a water-soluble packaging film material.
1.5 Research content
The research contents are as follows:
1) The HPMC water-soluble packaging film was prepared by solution casting film-forming method, and the properties of the film were analyzed to study the influence of the concentration of HPMC film-forming liquid and the film-forming temperature on the performance of HPMC water-soluble packaging film.
2) To study the effects of glycerol and sorbitol plasticizers on the mechanical properties, water solubility and optical properties of HPMC water-soluble packaging films.
3) To study the effect of glutaraldehyde cross-linking agent on the water solubility, mechanical properties and optical properties of HPMC water-soluble packaging films.
4) Preparation of AOB/HPMC water-soluble packaging film. The oxidation resistance, water solubility, mechanical properties and optical properties of AOB/HPMC thin films were studied.
Chapter 2 Preparation and Properties of Hydroxypropyl Methyl Cellulose Water-soluble Packaging Film
2.1 Introduction
Hydroxypropyl methylcellulose is a natural cellulose derivative. It is non-toxic, non-polluting, renewable, chemically stable, and has good water solubility and film-forming properties. It is a potential water-soluble packaging film material.
This chapter will use hydroxypropyl methylcellulose as raw material to prepare hydroxypropyl methylcellulose solution with a mass fraction of 2% to 6%, prepare water-soluble packaging film by solution casting method, and study the film-forming liquid Effects of concentration and film-forming temperature on film mechanical, optical, and water-solubility properties. The crystalline properties of the film were characterized by X-ray diffraction, and the tensile strength, elongation at break, light transmittance and haze of the hydroxypropyl methylcellulose water-soluble packaging film were analyzed by tensile test, optical test and water-solubility test degree and water solubility.
2.2 Experimental Department
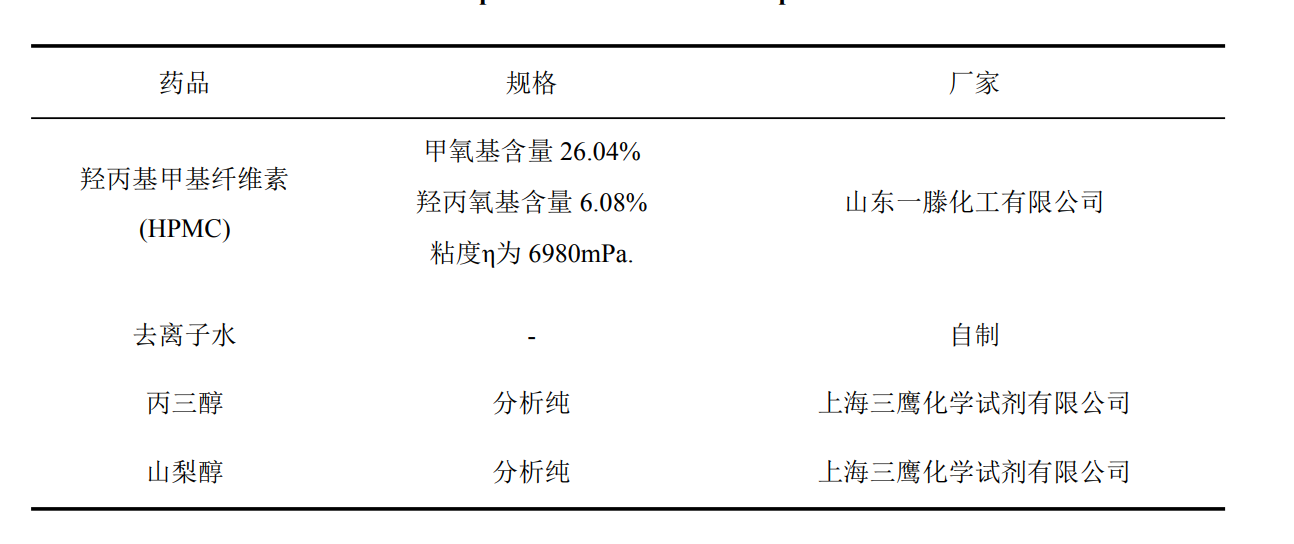
2.2.1 Experimental materials and instruments
2.2.2 Specimen Preparation
1) Weighing: Weigh a certain amount of hydroxypropyl methylcellulose with an electronic balance.
2) Dissolution: Add the weighed hydroxypropyl methylcellulose to the prepared deionized water, stir at normal temperature and pressure until it is completely dissolved, and then let it stand for a certain period of time (defoaming) to obtain a certain concentration of composition. membrane fluid. Formulated at 2%, 3%, 4%, 5% and 6%.
3) Film formation: ① Preparation of films with different film-forming concentrations: Inject HPMC film-forming solutions of different concentrations into glass petri dishes to cast films, and place them in a blast drying oven at 40~50°C to dry and form films. A hydroxypropyl methylcellulose water-soluble packaging film with a thickness of 25-50 μm is prepared, and the film is peeled off and placed in a drying box for use. ②Preparation of thin films at different film-forming temperatures (temperatures during drying and film-forming): Inject the film-forming solution with a concentration of 5% HPMC into a glass petri dish and cast films at different temperatures (30~70°C) The film was dried in a forced air drying oven. The hydroxypropyl methylcellulose water-soluble packaging film with a thickness of about 45 μm was prepared, and the film was peeled off and placed in a drying box for use. The prepared hydroxypropyl methylcellulose water-soluble packaging film is referred to as HPMC film for short.
2.2.3 Characterization and performance measurement
2.2.3.1 Wide-angle X-ray diffraction (XRD) analysis
Wide-angle X-ray diffraction (XRD) analyzes the crystalline state of a substance at the molecular level. The X-ray diffractometer of ARL/XTRA type produced by Thermo ARL Company in Switzerland was used for the determination. Measurement conditions: The X-ray source was a nickel-filtered Cu-kα line (40kV, 40mA). The scan angle is from 0° to 80° (2θ). Scanning speed 6°/min.
2.2.3.2 Mechanical properties
The tensile strength and elongation at break of the film are used as the criteria for judging its mechanical properties, and the tensile strength (Tensile Strength) refers to the stress when the film produces the maximum uniform plastic deformation, and the unit is MPa. Elongation at break (Breaking Elongation) refers to the ratio of the elongation when the film is broken to the original length, expressed in %. Using the INSTRON (5943) type miniature electronic universal tensile testing machine of Instron (Shanghai) testing equipment, according to GB13022-92 test method for tensile properties of plastic films, test at 25°C, 50%RH conditions, select Samples with uniform thickness and clean surface without impurities are tested.
2.2.3.3 Optical properties
Optical properties are an important indicator of the transparency of packaging films, mainly including the transmittance and haze of the film. The transmittance and haze of the films were measured using a transmittance haze tester. Pick a test sample with a clean surface and no creases, gently place it on the test stand, fix it with a suction cup, and measure the light transmittance and haze of the film at room temperature (25°C and 50%RH). The sample is tested 3 times and the average value is taken.
2.2.3.4 Water solubility
Cut a 30mm×30mm film with a thickness of about 45μm, add 100mL of water to a 200ml beaker, place the film in the center of the still water surface, and measure the time for the film to disappear completely [56]. Each sample was measured 3 times and the average value was taken, and the unit was min.
2.2.4 Data processing
The experimental data were processed by Excel and plotted by Origin software.
2.3 Results and Discussion
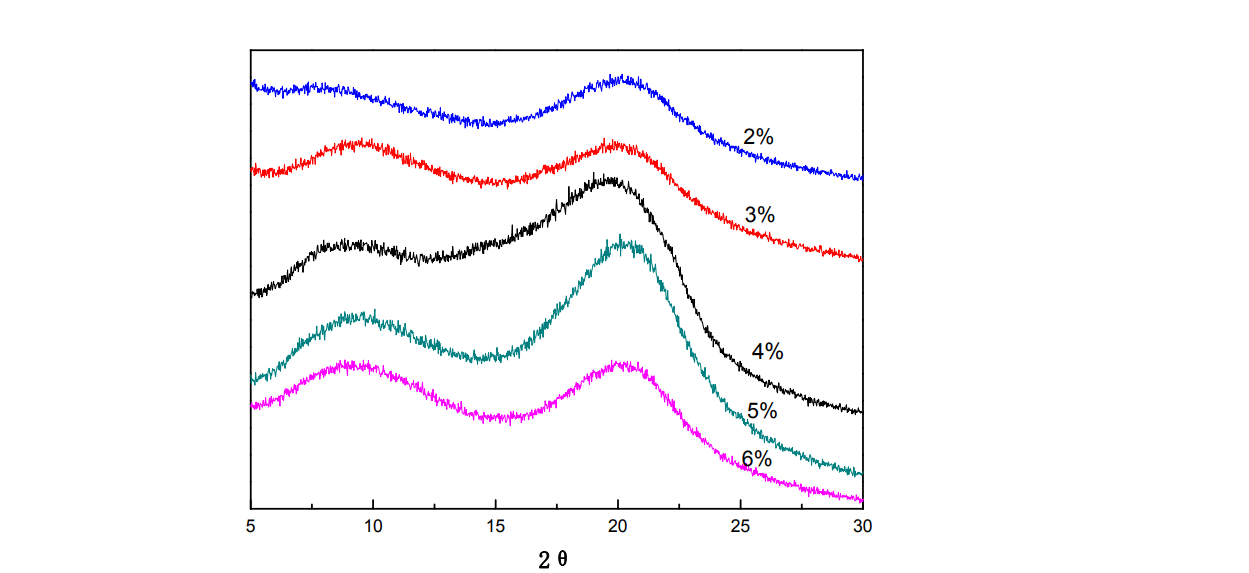
2.3.1.1 XRD patterns of HPMC thin films under different film-forming solution concentrations
Fig.2.1 XRD of HPMC films under different content of HP
Wide-angle X-ray diffraction is the analysis of the crystalline state of substances at the molecular level. Figure 2.1 is the XRD diffraction pattern of HPMC thin films under different film-forming solution concentrations. There are two diffraction peaks [57-59] (near 9.5° and 20.4°) in the HPMC film in the figure. It can be seen from the figure that with the increase of the HPMC concentration, the diffraction peaks of the HPMC film around 9.5° and 20.4° are first enhanced. and then weakened, the degree of molecular arrangement (ordered arrangement) first increased and then decreased. When the concentration is 5%, the orderly arrangement of HPMC molecules is optimal. The reason for the above phenomenon may be that with the increase of HPMC concentration, the number of crystal nuclei in the film-forming solution increases, thus making the HPM molecular arrangement more regular. When the HPMC concentration exceeds 5%, the XRD diffraction peak of the film weakens. From the point of view of molecular chain arrangement, when the HPMC concentration is too large, the viscosity of the film-forming solution is too high, making it difficult for the molecular chains to move and cannot be arranged in time, thus causing the degree of ordering of the HPMC films decreased.
2.3.1.2 Mechanical properties of HPMC thin films under different film-forming solution concentrations.
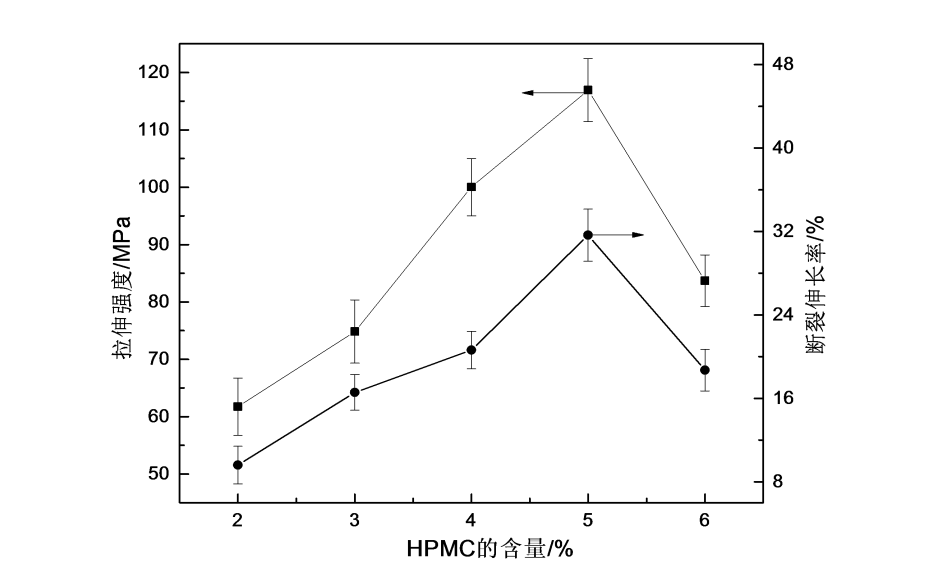
The tensile strength and elongation at break of the film are used as the criteria for judging its mechanical properties, and the tensile strength refers to the stress when the film produces the maximum uniform plastic deformation. The elongation at break is the ratio of the displacement to the original length of the film at break. The measurement of the mechanical properties of the film can judge its application in some fields.
Fig.2.2 The effect of different content of HPMC on mechanical properties of HPMC films
From Fig. 2.2, the changing trend of tensile strength and elongation at break of HPMC film under different concentrations of film-forming solution, it can be seen that the tensile strength and elongation at break of HPMC film increased first with the increase of concentration of HPMC film-forming solution. When the solution concentration is 5%, the mechanical properties of HPMC films are better. This is because when the film-forming liquid concentration is low, the solution viscosity is low, the interaction between molecular chains is relatively weak, and the molecules cannot be arranged in an orderly manner, so the crystallization ability of the film is low and its mechanical properties are poor; when the film-forming liquid concentration is 5 %, the mechanical properties reach the optimum value; as the concentration of the film-forming liquid continues to increase, the casting and diffusion of the solution become more difficult, resulting in uneven thickness of the obtained HPMC film and more surface defects [60] , resulting in a decrease in the mechanical properties of HPMC films. Therefore, the concentration of 5% HPMC film-forming solution is the most suitable. The performance of the obtained film is also better.
2.3.1.3 Optical properties of HPMC thin films under different film-forming solution concentrations
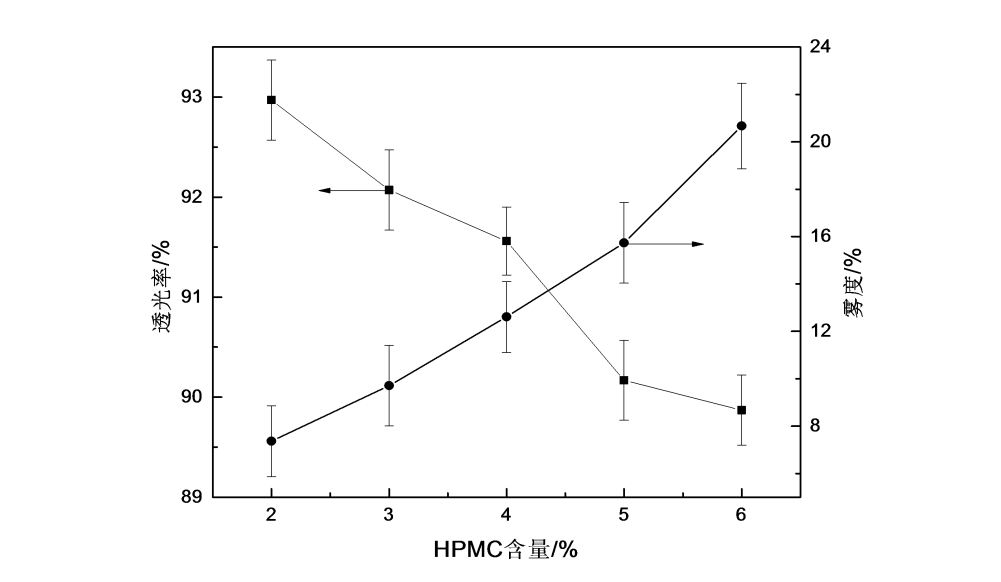
In packaging films, light transmittance and haze are important parameters indicating the transparency of the film. Figure 2.3 shows the changing trends of transmittance and haze of HPMC films under different film-forming liquid concentrations. It can be seen from the figure that with the increase of the concentration of the HPMC film-forming solution, the transmittance of the HPMC film gradually decreased, and the haze increased significantly with the increase of the concentration of the film-forming solution.
Fig.2.3 The effect of different content of HPMC on optical property of HPMC films
There are two main reasons: First, from the perspective of the number concentration of the dispersed phase, when the concentration is low, the number concentration has a dominant effect on the optical properties of the material [61]. Therefore, with the increase of the concentration of the HPMC film-forming solution, the film’s densities are reduced. The light transmittance decreased significantly, and the haze increased significantly. Second, from the analysis of the film-making process, it may be because the film was made by the solution casting film-forming method. The increase in the difficulty of elongation leads to the decrease of the smoothness of the film surface and the decrease of the optical properties of the HPMC film.
2.3.1.4 Water solubility of HPMC thin films under different film-forming liquid concentrations
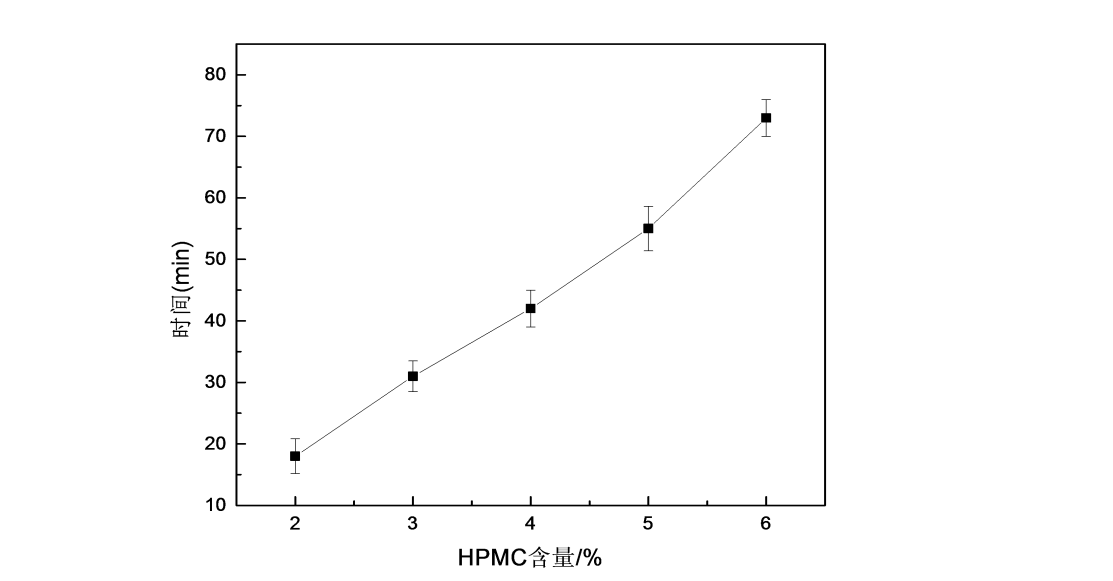
The water solubility of water-soluble films is related to their film-forming concentration. Cut out 30mm × 30mm films made with different film forming concentrations, and mark the film with “+” to measure the time for the film to disappear completely. If the film wraps or sticks to the walls of the beaker, retest. Figure 2.4 is the trend diagram of the water solubility of HPMC films under different film-forming liquid concentrations. It can be seen from the figure that with the increase of film-forming liquid concentration, the water-soluble time of HPMC films becomes longer, indicating that the water solubility of HPMC films decreases. It is speculated that the reason may be that with the increase of the concentration of the HPMC film-forming solution, the viscosity of the solution increases, and the intermolecular force strengthens after gelation, resulting in the weakening of the diffusivity of the HPMC film in water and the decrease in water solubility.
Fig.2.4 The effect of different content of HPMC on water solubility of HPMC films
2.3.2 Effect of film formation temperature on HPMC thin films
2.3.2.1 XRD patterns of HPMC thin films at different film forming temperatures
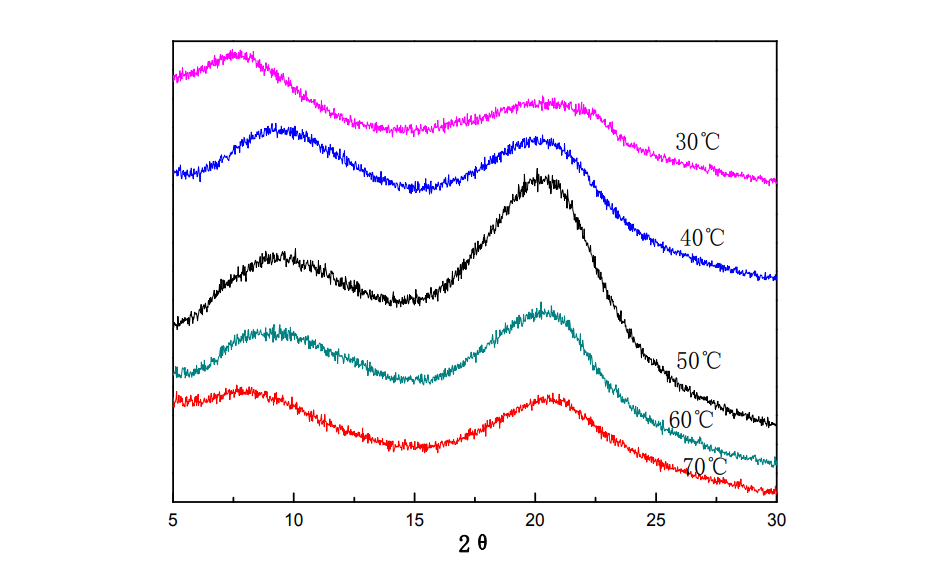
Fig.2.5 XRD of HPMC films under different film forming temperature
Figure 2.5 shows the XRD patterns of HPMC thin films at different film forming temperatures. Two diffraction peaks at 9.5° and 20.4° were analyzed for the HPMC film. From the perspective of the intensity of the diffraction peaks, with the increase of the film-forming temperature, the diffraction peaks at the two places first increased and then weakened, and the crystallization ability first increased and then decreased. When the film-forming temperature was 50 °C, the ordered arrangement of HPMC molecules From the perspective of the effect of temperature on homogeneous nucleation, when the temperature is low, the viscosity of the solution is high, the growth rate of crystal nuclei is small, and crystallization is difficult; as the film-forming temperature gradually increases, the rate of nucleation increases , the movement of the molecular chain is accelerated, the molecular chain is easily arranged around the crystal nucleus in an orderly manner, and it is easier to form crystallization, so the crystallization will reach the maximum value at a certain temperature; if the film-forming temperature is too high, the molecular motion is too violent, the formation of the crystal nucleus is difficult, and the formation of The nuclear efficiency is low and it is difficult to form crystals [62,63]. Therefore, the crystallinity of HPMC films increases first and then decreases with the increase of film forming temperature.
2.3.2.2 Mechanical properties of HPMC thin films at different film forming temperatures
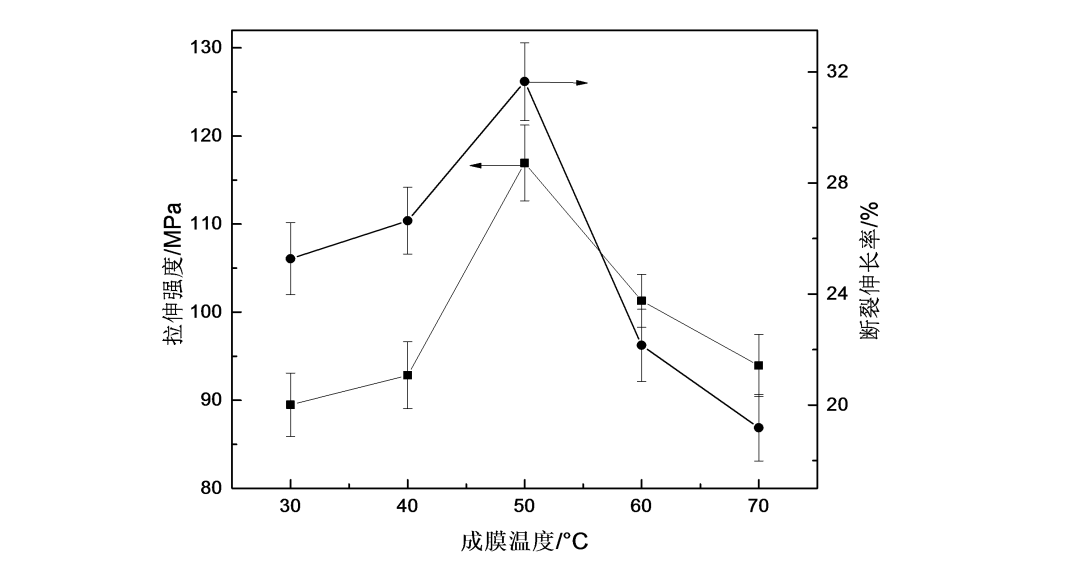
The change of the film forming temperature will have a certain degree of influence on the mechanical properties of the film. Figure 2.6 shows the changing trend of tensile strength and elongation at break of HPMC films at different film forming temperatures. At the same time, it showed a trend of increasing first and then decreasing. When the film forming temperature was 50 °C, the tensile strength and elongation at break of the HPMC film reached the maximum values, which were 116 MPa and 32%, respectively.
Fig.2.6 The effect of film forming temperature on mechanical properties of HPMC films
From the perspective of molecular arrangement, the greater the orderly arrangement of molecules, the better the tensile strength [64]. From Fig. 2.5 XRD patterns of HPMC films at different film formation temperatures, it can be seen that with the increase of film formation temperature, the orderly arrangement of HPMC molecules first increases and then decreases. When the film formation temperature is 50 °C, the The degree of ordered arrangement is the largest, so the tensile strength of HPMC films first increases and then decreases with the increase of the film forming temperature, and the maximum value appears at the film forming temperature of 50℃. The elongation at break shows a trend of increasing first and then decreasing. The reason may be that with the increase of temperature, the orderly arrangement of molecules first increases and then decreases, and the crystalline structure formed in the polymer matrix is dispersed in the uncrystallized polymer matrix. In the matrix, a physical cross-linked structure is formed, which plays a certain role in toughening [65], thereby promoting the elongation at break of the HPMC film to appear a peak at the film formation temperature of 50 °C.
2.3.2.3 Optical properties of HPMC films at different film forming temperatures
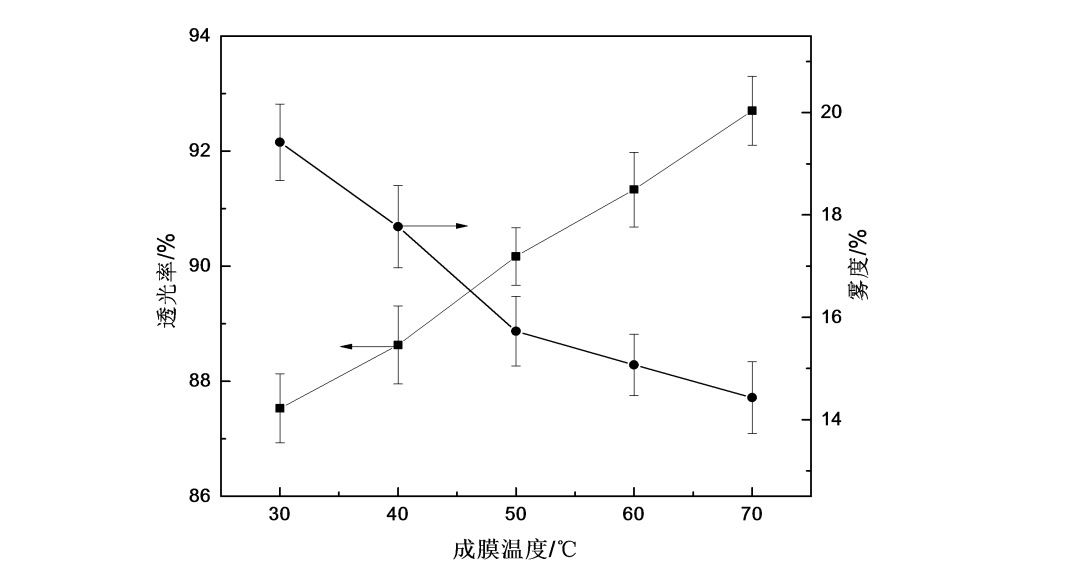
Figure 2.7 is the change curve of the optical properties of HPMC films at different film forming temperatures. It can be seen from the figure that with the increase of film forming temperature, the transmittance of HPMC film gradually increases, the haze gradually decreases, and the optical properties of HPMC film gradually become better.
Fig.2.7 The effect of film forming temperature on optical property of HPMC
According to the influence of temperature and water molecules on the film [66], when the temperature is low, water molecules exist in HPMC in the form of bound water, but this bound water will gradually volatilize, and HPMC is in a glass state. The volatilization of the film forms holes in HPMC, and then scattering is formed at the holes after light irradiation [67], so the light transmittance of the film is low and the haze is high; as the temperature increases, the molecular segments of HPMC begin to move, The holes formed after the volatilization of water are filled, the holes gradually decrease, the degree of light scattering at the holes decreases, and the transmittance increases [68], so the light transmittance of the film increases and the haze decreases.
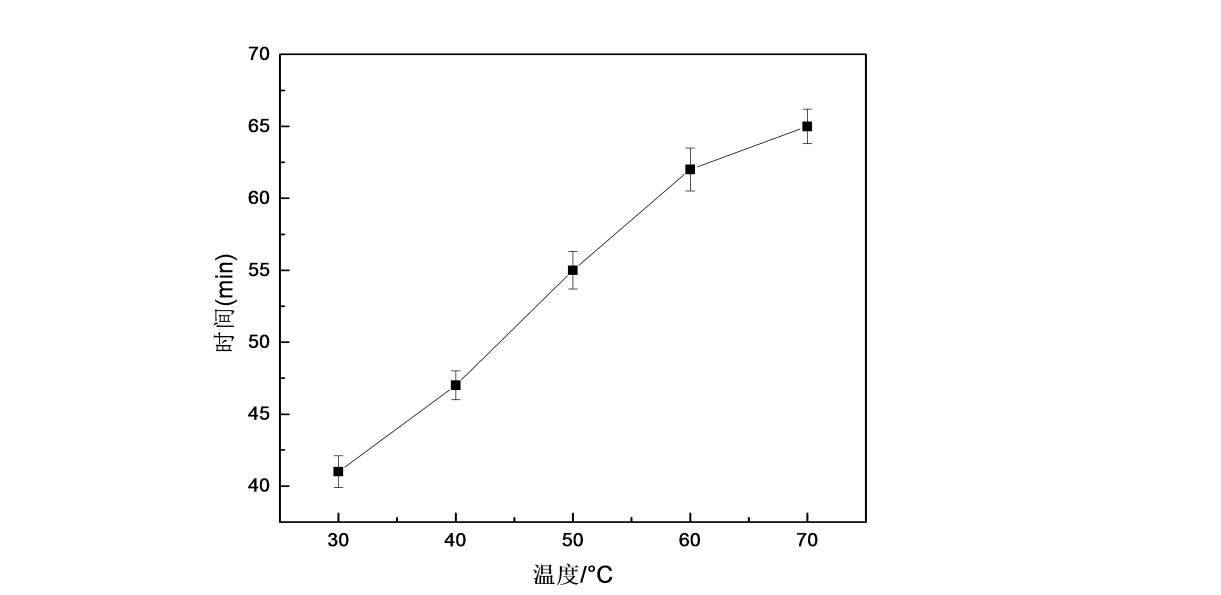
2.3.2.4 Water solubility of HPMC films at different film forming temperatures
Figure 2.8 shows the water solubility curves of HPMC films at different film forming temperatures. It can be seen from the figure that the water solubility time of HPMC films increases with the increase of film forming temperature, that is, the water solubility of HPMC films becomes worse. With the increase of film-forming temperature, the evaporation rate of water molecules and the gelation rate are accelerated, the movement of molecular chains is accelerated, the molecular spacing is reduced, and the molecular arrangement on the surface of the film is more dense, which makes it difficult for water molecules to enter between HPMC molecules. Water solubility is also reduced.
Fig.2.8 The effect of film forming temperature on water solubility of HPMC film
2.4 Summary of this chapter
In this chapter, hydroxypropyl methylcellulose was used as raw material to prepare HPMC water-soluble packaging film by solution casting film-forming method. The crystallinity of the HPMC film was analyzed by XRD diffraction; the mechanical properties of the HPMC water-soluble packaging film were tested and analyzed by a micro-electronic universal tensile testing machine, and the optical properties of the HPMC film were analyzed by a light transmission haze tester. The dissolution time in water (water solubility time) is used to analyze its water solubility. The following conclusions are drawn from the above research:
1) The mechanical properties of HPMC films first increased and then decreased with the increase of the concentration of the film-forming solution, and firstly increased and then decreased with the increase of the film-forming temperature. When the concentration of the HPMC film-forming solution was 5% and the film-forming temperature was 50 °C, the mechanical properties of the film are good. At this time, the tensile strength is about 116MPa, and the elongation at break is about 31%;
2) The optical properties of HPMC films decrease with the increase of the concentration of the film-forming solution, and gradually increase with the increase of the film-forming temperature; comprehensively consider that the concentration of the film-forming solution should not exceed 5%, and the film-forming temperature should not exceed 50°C
3) The water solubility of HPMC films showed a downward trend with the increase of the concentration of the film-forming solution and the increase of the film-forming temperature. When the concentration of 5% HPMC film-forming solution and the film-forming temperature of 50 °C were used, the water-dissolving time of the film was 55 min.
Chapter 3 Effects of Plasticizers on HPMC Water-Soluble Packaging Films
3.1 Introduction
As a new type of natural polymer material HPMC water-soluble packaging film has a good development prospect. Hydroxypropyl methylcellulose is a natural cellulose derivative. It is non-toxic, non-polluting, renewable, chemically stable, and has good properties. Water-soluble and film-forming, it is a potential water-soluble packaging film material.
The previous chapter discussed the preparation of HPMC water-soluble packaging film by using hydroxypropyl methylcellulose as raw material by solution casting film-forming method, and the effect of film-forming liquid concentration and film-forming temperature on hydroxypropyl methylcellulose water-soluble packaging film. performance impact. The results show that the tensile strength of the film is about 116MPa and the elongation at break is 31% under the optimum concentration and process conditions. The toughness of such films is poor in some applications and needs further improvement.
In this chapter, hydroxypropyl methylcellulose is still used as raw material, and the water-soluble packaging film is prepared by solution casting film-forming method. , elongation at break), optical properties (transmittance, haze) and water solubility.
3.2 Experimental Department
3.2.1 Experimental materials and instruments
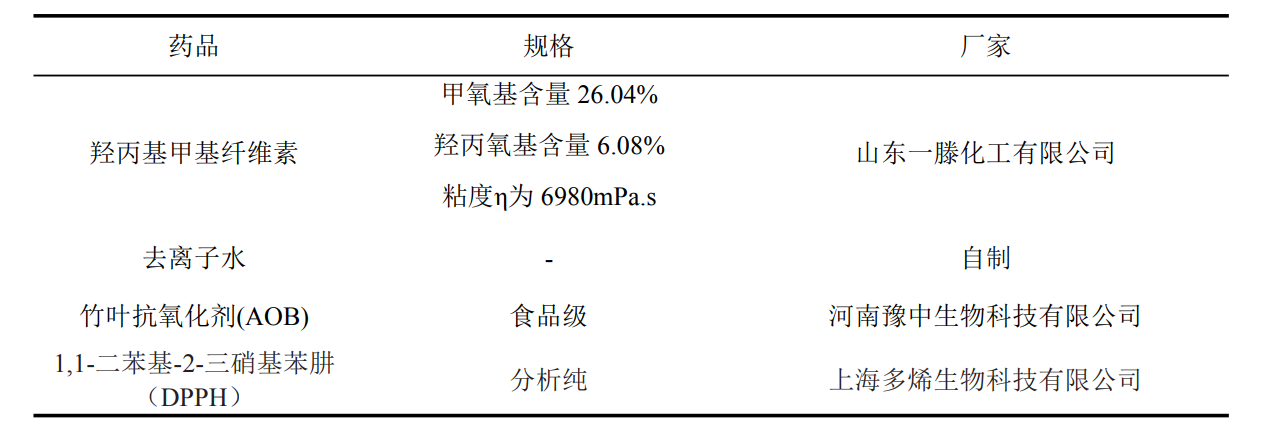
Table 3.1 Experimental materials and specifications
Table 3.2 Experimental instruments and specifications
3.2.2 Sample preparation
1) Weighing: Weigh a certain amount of hydroxypropyl methylcellulose (5%) and sorbitol (0.05%, 0.15%, 0.25%, 0.35%, 0.45%) with an electronic balance, and use a syringe to measure glycerol Alcohol (0.05%, 0.15%, 0.25%, 0.35%, 0.45%).
2) Dissolution: Add the weighed hydroxypropyl methylcellulose into the prepared deionized water, stir at normal temperature and pressure until it is completely dissolved, and then add glycerol or sorbitol in different mass fractions respectively. In the hydroxypropyl methylcellulose solution, stir for a period of time to make it evenly mixed, and let it stand for 5 minutes (defoaming) to obtain a certain concentration of film-forming liquid.
3) Film making: inject the film-forming liquid into a glass petri dish and cast it to form a film, let it stand for a certain period of time to make it gel, and then put it in a blast drying oven to dry and form a film to make a film with a thickness of 45 μm. After the film is placed in a drying box for use.
3.2.3 Characterization and performance testing
3.2.3.1 Infrared absorption spectroscopy (FT-IR) analysis
Infrared absorption spectroscopy (FTIR) is a powerful method to characterize the functional groups contained in the molecular structure and to identify functional groups. The infrared absorption spectrum of the HPMC packaging film was measured using a Nicolet 5700 Fourier transform infrared spectrometer produced by Thermoelectric Corporation. The thin film method was used in this experiment, the scanning range was 500-4000 cm-1, and the number of scanning was 32. The sample films were dried in a drying oven at 50 °C for 24 h for infrared spectroscopy.
3.2.3.2 Wide-angle X-ray diffraction (XRD) analysis: same as 2.2.3.1
3.2.3.3 Determination of mechanical properties
The tensile strength and elongation at break of the film are used as parameters for judging its mechanical properties. The elongation at break is the ratio of the displacement to the original length when the film is broken, in %. Using the INSTRON (5943) miniature electronic universal tensile testing machine of Instron (Shanghai) testing equipment, in accordance with GB13022-92 test method for tensile properties of plastic films, test at 25 ° C, 50% RH conditions, select Samples with uniform thickness and clean surface without impurities are tested.
3.2.3.4 Determination of optical properties: same as 2.2.3.3
3.2.3.5 Determination of water solubility
Cut a 30mm×30mm film with a thickness of about 45μm, add 100mL of water to a 200ml beaker, place the film in the center of the still water surface, and measure the time for the film to disappear completely [56]. Each sample was measured 3 times and the average value was taken, and the unit was min.
3.2.4 Data processing
The experimental data was processed by Excel, and the graph was drawn by Origin software.
3.3 Results and Discussion
3.3.1 Effects of glycerol and sorbitol on the infrared absorption spectrum of HPMC films
(a) Glycerol (b) Sorbitol
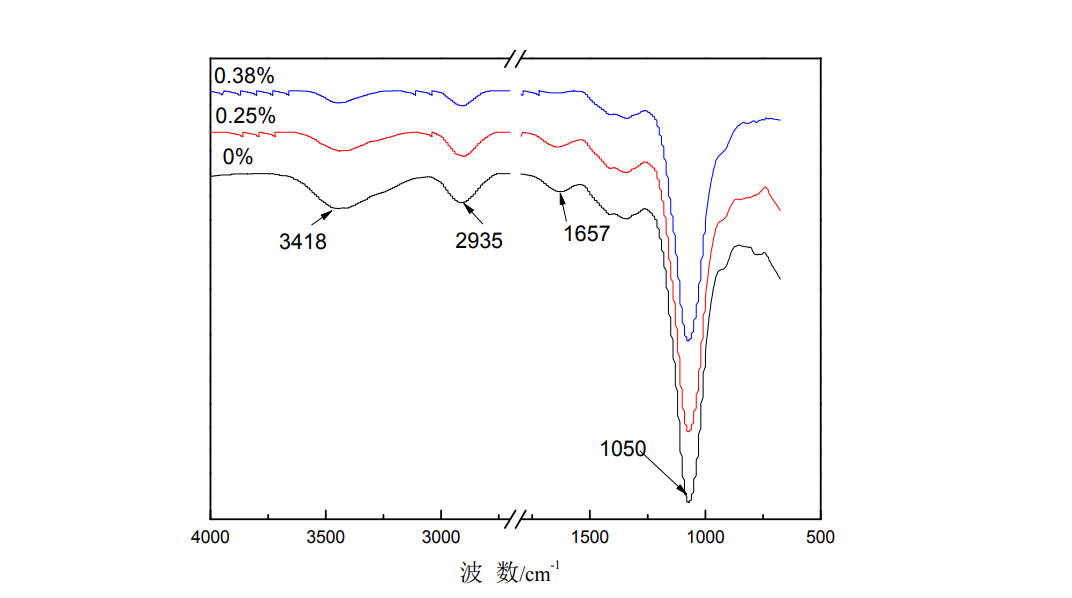
Fig.3.1 FT-IR of the HPMC films under different glycerol or sorbitolum concentrat
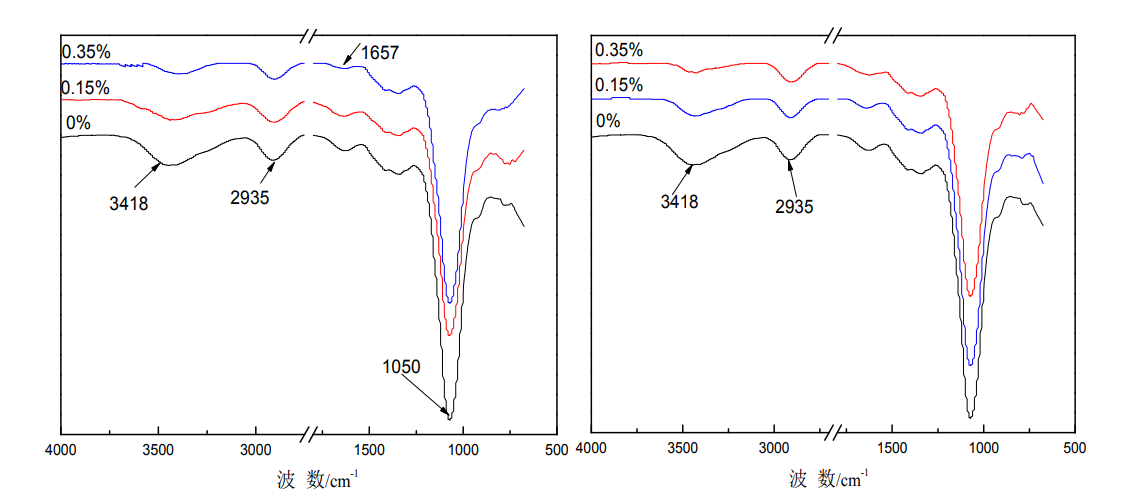
Infrared absorption spectroscopy (FTIR) is a powerful method to characterize the functional groups contained in the molecular structure and to identify functional groups. Figure 3.1 shows the infrared spectra of HPMC films with different glycerol and sorbitol additions. It can be seen from the figure that the characteristic skeleton vibration peaks of HPMC films are mainly in the two regions: 2600~3700cm-1 and 750~1700cm-1 [57-59], 3418cm-1
The nearby absorption bands are caused by the stretching vibration of the O-H bond, 2935cm-1 is the absorption peak of -CH2, 1050cm-1 is the absorption peak of -C-O- and -C-O-C- on the primary and secondary hydroxyl groups, and 1657cm-1 is the absorption peak of the hydroxypropyl group. The absorption peak of the hydroxyl group in the stretching vibration of the framework, 945cm-1 is the rocking absorption peak of -CH3 [69]. The absorption peaks at 1454cm-1, 1373cm-1, 1315cm-1 and 945cm-1 are assigned to the asymmetric, symmetrical deformation vibrations, in-plane and out-of-plane bending vibrations of -CH3, respectively [18]. After plasticization, no new absorption peaks appeared in the infrared spectrum of the film, indicating that HPMC did not undergo essential changes, that is, the plasticizer did not destroy its structure. With the addition of glycerol, the stretching vibration peak of -OH at 3418cm-1 of HPMC film weakened, and the absorption peak at 1657cm-1, the absorption peaks at 1050cm-1 weakened, and the absorption peaks of -C-O- and -C-O-C- on the primary and secondary hydroxyl groups weakened; with the addition of sorbitol to the HPMC film, the -OH stretching vibration peaks at 3418cm-1 weakened, and the absorption peaks at 1657cm-1 weakened. . The changes of these absorption peaks are mainly caused by inductive effects and intermolecular hydrogen bonding, which make them change with the adjacent -CH3 and -CH2 bands. due to small, the insertion of molecular substances hinders the formation of intermolecular hydrogen bonds, so the tensile strength of the plasticized film decreases [70].
3.3.2 Effects of glycerol and sorbitol on the XRD patterns of HPMC films
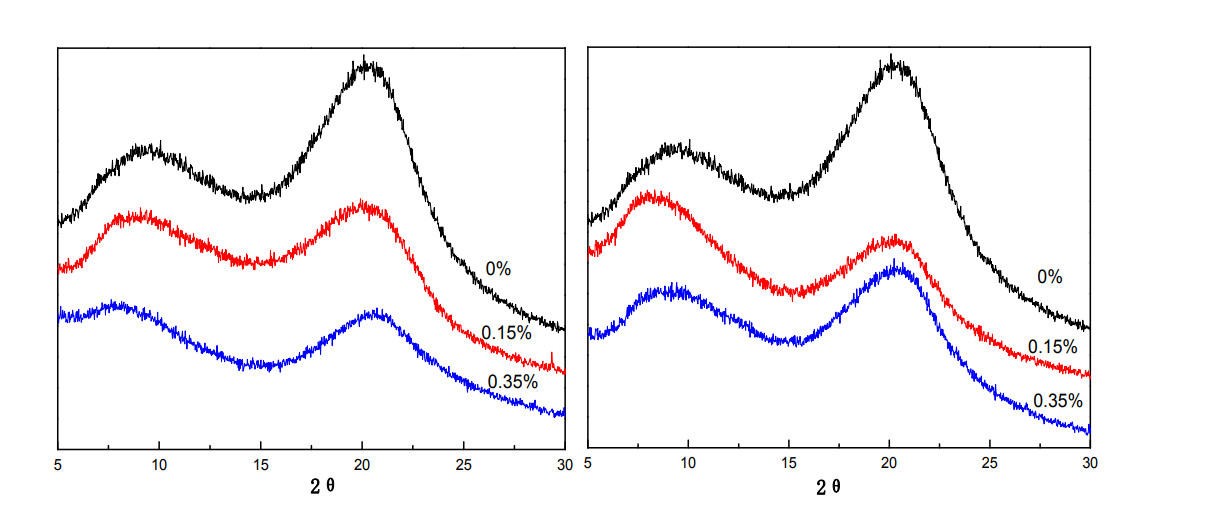
(a) Glycerol (b) Sorbitol
Fig.3.2 XRD of HPMC films under different glycerol or sorbitolum concentra
Wide-angle X-ray diffraction (XRD) analyzes the crystalline state of substances at the molecular level. The X-ray diffractometer of ARL/XTRA type produced by Thermo ARL Company in Switzerland was used for the determination. Figure 3.2 is the XRD patterns of HPMC films with different additions of glycerol and sorbitol. With the addition of glycerol, the intensity of the diffraction peaks at 9.5° and 20.4° both weakened; with the addition of sorbitol, when the addition amount was 0.15%, the diffraction peak at 9.5° was enhanced, and the diffraction peak at 20.4° was weakened, but the total The diffraction peak intensity was lower than that of the HPMC film without sorbitol. With the continuous addition of sorbitol, the diffraction peak at 9.5° weakened again, and the diffraction peak at 20.4° did not change significantly. This is because the addition of small molecules of glycerol and sorbitol disturbs the orderly arrangement of molecular chains and destroys the original crystal structure, thereby reducing the crystallization of the film. It can be seen from the figure that glycerol has a great influence on the crystallization of HPMC films, indicating that glycerol and HPMC have good compatibility, while sorbitol and HPMC have poor compatibility. From the structural analysis of plasticizers, sorbitol has a sugar ring structure similar to that of cellulose, and its steric hindrance effect is large, resulting in weak interpenetration between sorbitol molecules and cellulose molecules, so it has little effect on cellulose crystallization.
[48].
3.3.3 Effects of glycerol and sorbitol on the mechanical properties of HPMC films
The tensile strength and elongation at break of the film are used as parameters to judge its mechanical properties, and the measurement of mechanical properties can judge its application in certain fields. Figure 3.3 shows the change in tensile strength and elongation at break of HPMC films after adding plasticizers.
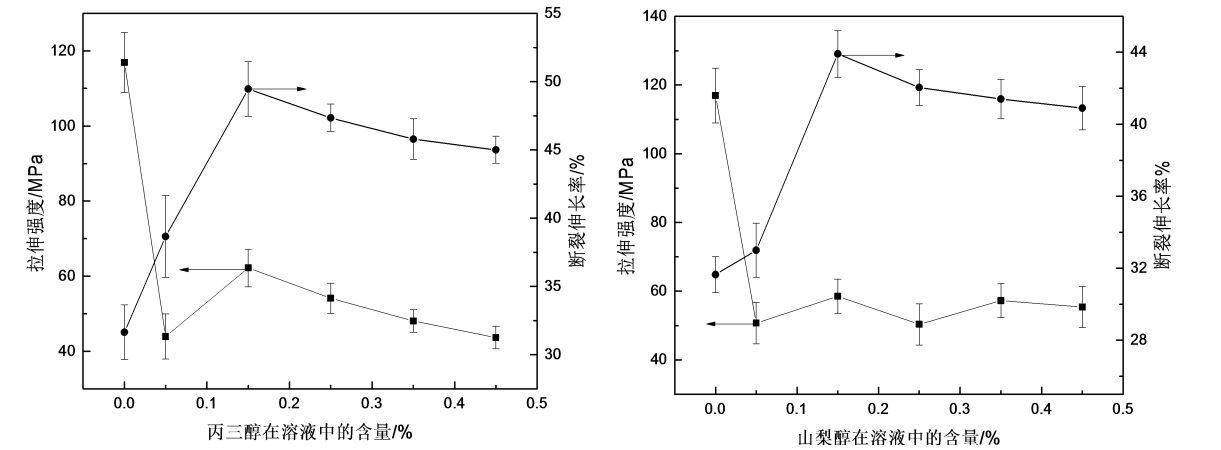
Fig.3.3 The effect of glycerol or sorbitolumon on machine properties of HPMC films
It can be seen from Figure 3.3(a) that with the addition of glycerol, the elongation at break of the HPMC film first increases and then decreases, while the tensile strength first decreases rapidly, then increases slowly and then continues to decrease. The elongation at break of HPMC film first increased and then decreased, because glycerol has more hydrophilic groups, which makes the material and water molecules have a strong hydration effect [71], thus improving the flexibility of the film. With the continuous increase of glycerol addition, the elongation at break of HPMC film decreases, this is because glycerol makes the HPMC molecular chain gap larger, and the entanglement between macromolecules The point is reduced, and the film is prone to break when the film is stressed, thereby reducing the elongation at break of the film. The reason for the rapid decrease of tensile strength is: the addition of small molecules of glycerol disturbs the close arrangement between the HPMC molecular chains, weakens the interaction force between macromolecules, and reduces the tensile strength of the film; the tensile strength A small increase, from the perspective of molecular chain arrangement, appropriate glycerol increases the flexibility of HPMC molecular chains to a certain extent, promotes the arrangement of polymer molecular chains, and makes the tensile strength of the film increase slightly; However, when there is too much glycerol, the molecular chains are de-arranged at the same time as the orderly arrangement, and the rate of de-arrangement is higher than that of the ordered arrangement [72], which reduces the crystallization of the film, resulting in low tensile strength of the HPMC film. Since the toughening effect is at the expense of the tensile strength of the HPMC film, the amount of glycerol added should not be too much.
As shown in Figure 3.3(b), with the addition of sorbitol, the elongation at break of the HPMC film first increased and then decreased. When the amount of sorbitol was 0.15%, the elongation at break of the HPMC film reached 45%, and then the elongation at break of the film gradually decreased again. The tensile strength decreases rapidly, and then fluctuates around 50MP with the continuous addition of sorbitol. It can be seen that when the amount of sorbitol added is 0.15%, the plasticizing effect is the best. This is because the addition of small molecules of sorbitol disturbs the regular arrangement of molecular chains, making the gap between molecules larger, the interaction force is reduced, and the molecules are easy to slide, so the elongation at break of the film increases and the tensile strength decline. As the amount of sorbitol continued to increase, the elongation at break of the film decreased again, because the small molecules of sorbitol were fully dispersed between the macromolecules, resulting in the gradual reduction of the entanglement points between the macromolecules and the decrease in the elongation at break of the film.
Comparing the plasticizing effects of glycerol and sorbitol on HPMC films, adding 0.15% glycerol can increase the elongation at break of the film to about 50%; while adding 0.15% sorbitol can only increase the elongation at break of the film The rate reaches about 45%. Tensile strength decreased, and the decrease was smaller when glycerol was added. It can be seen that the plasticizing effect of glycerol on HPMC film is better than that of sorbitol.
3.3.4 Effects of glycerol and sorbitol on the optical properties of HPMC films
(a) Glycerol (b) Sorbitol
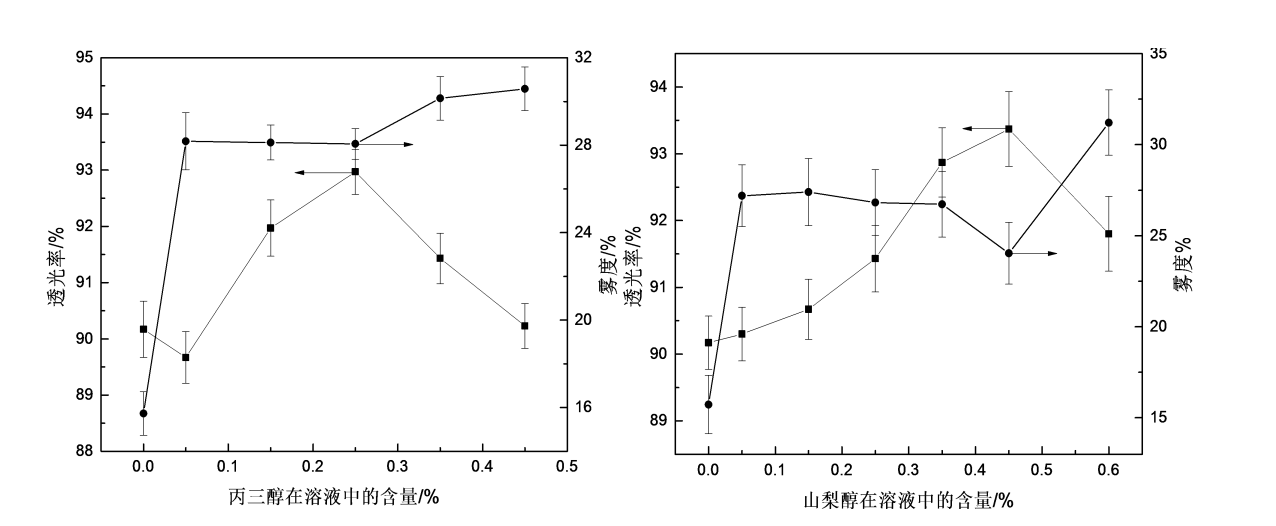
Fig.3.4 The effect of glycerol or sorbitolumon optical property of HPMC films
Light transmittance and haze are important parameters of the transparency of the packaging film. The visibility and clarity of the packaged goods mainly depend on the light transmittance and haze of the packaging film. As shown in Figure 3.4, the addition of glycerol and sorbitol both affected the optical properties of HPMC films, especially the haze. Figure 3.4(a) is a graph showing the effect of glycerol addition on the optical properties of HPMC films. With the addition of glycerol, the transmittance of HPMC films first increased and then decreased, reaching a maximum value around 0.25%; The haze increased rapidly and then slowly. It can be seen from the above analysis that when the addition amount of glycerol is 0.25%, the optical properties of the film are better, so the addition amount of glycerol should not exceed 0.25%. Figure 3.4(b) is a graph showing the effect of sorbitol addition on the optical properties of HPMC films. It can be seen from the figure that with the addition of sorbitol, the haze of HPMC films increases first, then decreases slowly and then increases, and the transmittance increases first and then increases. decreased, and the light transmittance and haze appeared peaks at the same time when the amount of sorbitol was 0.45%. It can be seen that when the amount of sorbitol added is between 0.35 and 0.45%, its optical properties are better. Comparing the effects of glycerol and sorbitol on the optical properties of HPMC films, it can be seen that sorbitol has little effect on the optical properties of the films.
Generally speaking, materials with high light transmittance will have lower haze, and vice versa, but this is not always the case. Some materials have high light transmittance but also high haze values, such as thin films like frosted glass [73]. The film prepared in this experiment can choose the appropriate plasticizer and addition amount according to the needs.
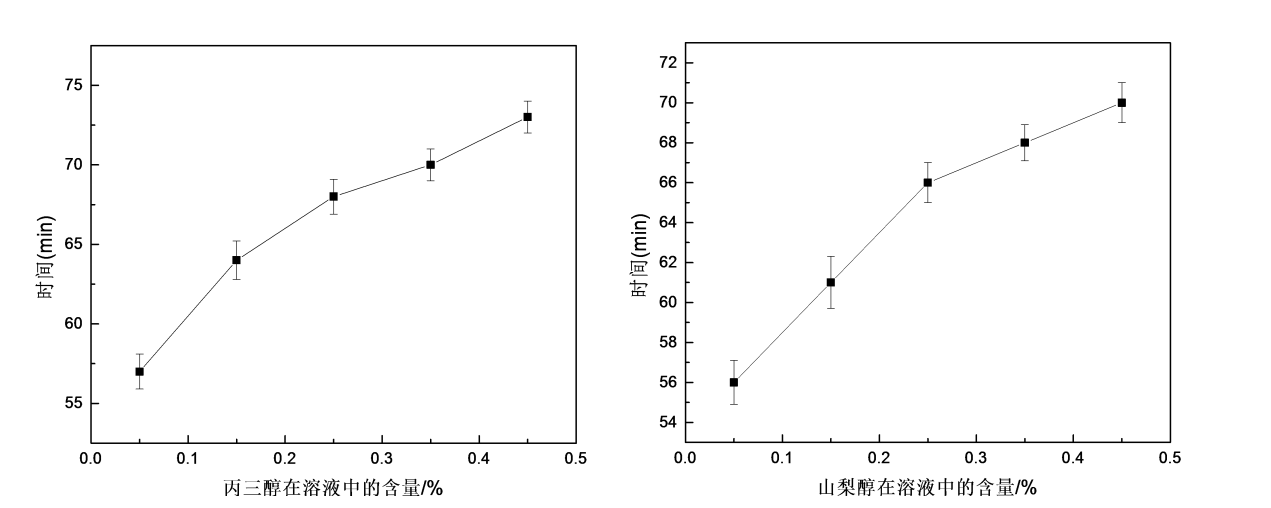
3.3.5 Effects of glycerol and sorbitol on the water solubility of HPMC films
(a) Glycerol (b)Sorbitol
Fig.3.5 The effect of glycerol or sorbitolumon water solubility of HPMC films
Figure 3.5 shows the effect of glycerol and sorbitol on the water solubility of HPMC films. It can be seen from the figure that with the increase of plasticizer content, the water solubility time of HPMC film is prolonged, that is, the water solubility of HPMC film gradually decreases, and glycerol has a greater impact on the water solubility of HPMC film than sorbitol. The reason why hydroxypropyl methylcellulose has good water solubility is because of the existence of a large number of hydroxyl groups in its molecule. From the analysis of the infrared spectrum, it can be seen that with the addition of glycerol and sorbitol, the hydroxyl vibration peak of the HPMC film weakens, indicating that the number of hydroxyl groups in the HPMC molecule decreases and the hydrophilic group decreases, so the water solubility of the HPMC film decreases.
3.4 Sections of this chapter
Through the above performance analysis of HPMC films, it can be seen that the plasticizers glycerol and sorbitol improve the mechanical properties of HPMC films and increase the elongation at break of the films. When the addition of glycerol is 0.15%, the mechanical properties of HPMC films are relatively good, the tensile strength is about 60MPa, and the elongation at break is about 50%; when the addition of glycerol is 0.25%, the optical properties are better. When the content of sorbitol is 0.15%, the tensile strength of HPMC film is about 55MPa, and the elongation at break increases to about 45%. When the content of sorbitol is 0.45%, the optical properties of the film are better. Both plasticizers reduced the water solubility of HPMC films, while sorbitol had less effect on the water solubility of HPMC films. The comparison of the effects of the two plasticizers on the properties of HPMC films shows that the plasticizing effect of glycerol on HPMC films is better than that of sorbitol.
Chapter 4 Effects of Crosslinking Agents on HPMC Water-Soluble Packaging Films
4.1 Introduction
Hydroxypropyl methylcellulose contains a lot of hydroxyl groups and hydroxypropoxy groups, so it has good water solubility. This paper uses its good water solubility to prepare a novel green and environmentally friendly water-soluble packaging film. Depending on the application of the water-soluble film, fast dissolution of the water-soluble film is required in most applications, but sometimes delayed dissolution is also desired [21].
Therefore, in this chapter, glutaraldehyde is used as the modified cross-linking agent for the water-soluble packaging film of hydroxypropyl methylcellulose, and its surface is cross-linked to modify the film to reduce the water-solubility of the film and delay the water-solubility time. The effects of different glutaraldehyde volume additions on the water solubility, mechanical properties and optical properties of hydroxypropyl methylcellulose films were mainly studied.
4.2 Experimental part
4.2.1 Experimental materials and instruments
Table 4.1 Experimental materials and specifications
4.2.2 Specimen Preparation
1) Weighing: weigh a certain amount of hydroxypropyl methylcellulose (5%) with an electronic balance;
2) Dissolution: the weighed hydroxypropyl methylcellulose is added to the prepared deionized water, stirred at room temperature and pressure until completely dissolved, and then different amounts of glutaraldehyde (0.19% 0.25% 0.31%, 0.38%, 0.44%), stirred evenly, let stand for a certain period of time (defoaming), and the film-forming liquid with different glutaraldehyde added amounts is obtained;
3) Film making: inject the film forming liquid into the glass Petri dish and cast the film, put it in the air drying box of 40 ~ 50 ° C to dry the film, make a film with a thickness of 45μm, uncover the film, and put it in the drying box for backup.
4.2.3 Characterization and performance testing
4.2.3.1 Infrared absorption spectroscopy (FT-IR) analysis
The infrared suction of HPMC films was determined using the Nicolet 5700 Fourier infrared spectrometer produced by the American Thermoelectric Company close the spectrum.
4.2.3.2 Wide-angle X-ray diffraction (XRD) analysis
Wide-angle X-ray diffraction (XRD) is the analysis of the crystallization state of a substance at the molecular level. In this paper, the crystallization state of the thin film was determined using an ARL/XTRA X-ray diffractometer produced by Thermo ARL of Switzerland. Measurement conditions: The X-ray source is a nickel filter Cu-kα line (40 kV, 40 mA). Scan angle from 0° to 80° (2θ). Scan speed 6°/min.
4.2.3.3 Determination of water solubility: same as 2.2.3.4
4.2.3.4 Determination of mechanical properties
Using the INSTRON (5943) miniature electronic universal tensile testing machine of Instron (Shanghai) testing equipment, according to GB13022-92 test method for tensile properties of plastic films, test at 25 ° C, 50% RH conditions, select Samples with uniform thickness and clean surface without impurities are tested.
4.2.3.5 Determination of optical properties
Using a light transmittance haze tester, select a sample to be tested with a clean surface and no creases, and measure the light transmittance and haze of the film at room temperature (25°C and 50%RH).
4.2.4 Data processing
The experimental data were processed by Excel and graphed by Origin software.
4.3 Results and Discussion
4.3.1 Infrared absorption spectra of glutaraldehyde-crosslinked HPMC films
Fig.4.1 FT-IR of HPMC films under different glutaraldehyde content
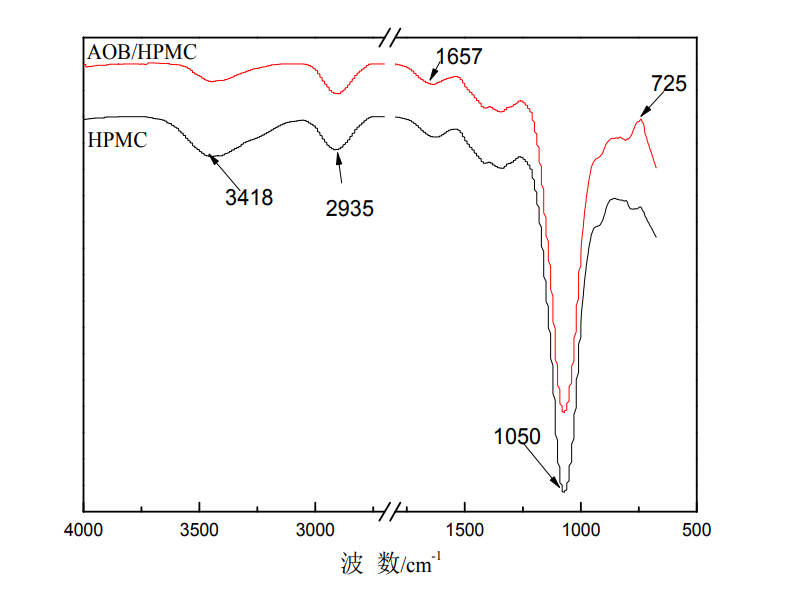
Infrared absorption spectroscopy is a powerful means to characterize the functional groups contained in the molecular structure and to identify functional groups. In order to further understand the structural changes of hydroxypropyl methylcellulose after modification, infrared tests were conducted on HPMC films before and after modification. Figure 4.1 shows the infrared spectra of HPMC films with different amounts of glutaraldehyde, and the deformation of HPMC films
The vibrational absorption peaks of -OH are near 3418cm-1 and 1657cm-1. Comparing the crosslinked and uncrosslinked infrared spectra of HPMC films, it can be seen that with the addition of glutaraldehyde, the vibrational peaks of -OH at 3418cm-1 and 1657cm- The absorption peak of hydroxyl group on 1 hydroxypropoxy group was significantly weakened, indicating that the number of hydroxyl groups in the HPMC molecule was reduced, which was caused by the cross-linking reaction between some hydroxyl groups of HPMC and the dialdehyde group on glutaraldehyde [74]. In addition, it was found that the addition of glutaraldehyde did not change the position of each characteristic absorption peak of HPMC, indicating that the addition of glutaraldehyde did not destroy the groups of HPMC itself.
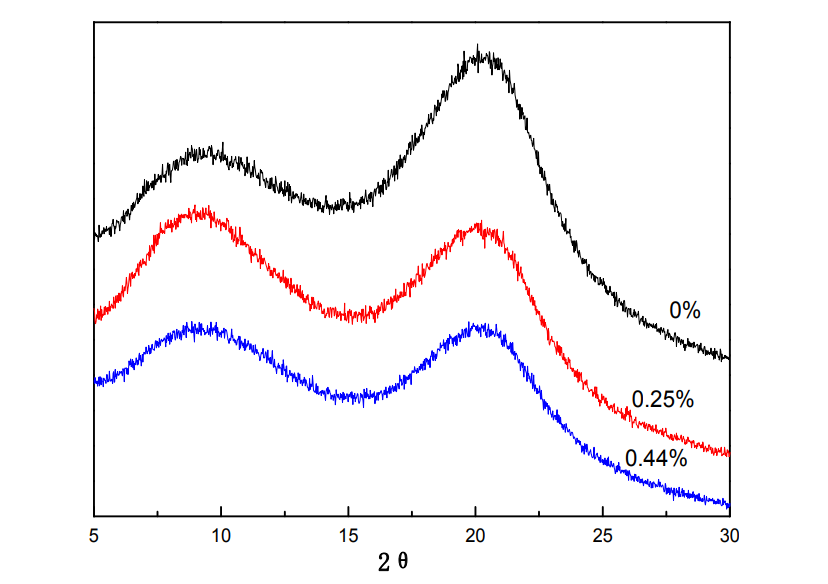
4.3.2 XRD patterns of glutaraldehyde-crosslinked HPMC films
By performing X-ray diffraction on a material and analyzing its diffraction pattern, it is a research method to obtain information such as the structure or morphology of atoms or molecules inside the material. Figure 4.2 shows the XRD patterns of HPMC films with different glutaraldehyde additions. With the increase of glutaraldehyde addition, the intensity of the diffraction peaks of HPMC around 9.5° and 20.4° weakened, because the aldehydes on the glutaraldehyde molecule weakened. The cross-linking reaction occurs between the hydroxyl group and the hydroxyl group on the HPMC molecule, which limits the mobility of the molecular chain [75], thereby reducing the orderly arrangement ability of the HPMC molecule.
Fig.4.2 XRD of HPMC films under different glutaraldehyde content
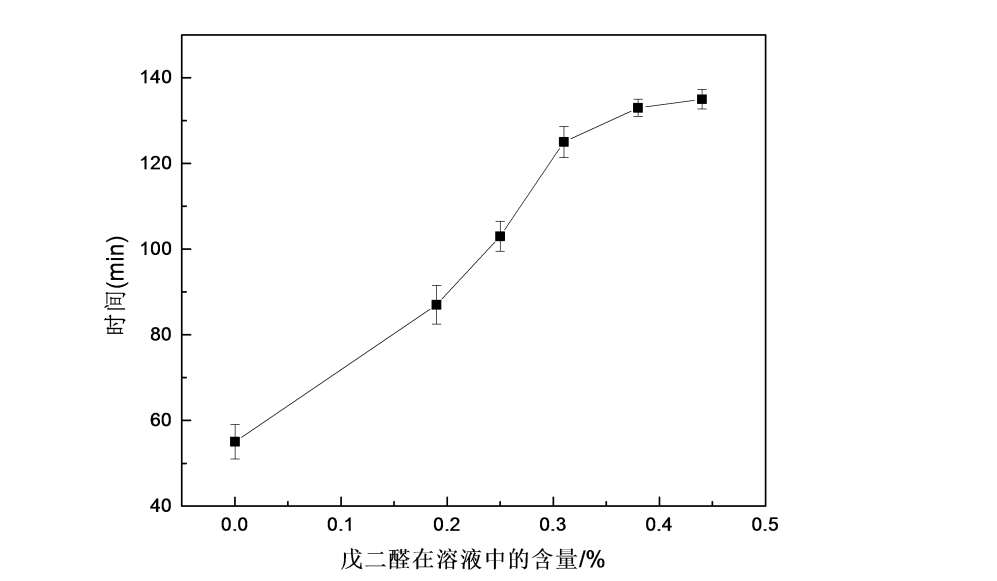
4.3.3 The effect of glutaraldehyde on the water solubility of HPMC films
Fig.4.3 The effect of glutaraldehyde on water solubility of HPMC films
From Figure 4.3 the effect of different glutaraldehyde additions on the water solubility of HPMC films, it can be seen that with the increase of glutaraldehyde dosage, the water solubility time of HPMC films is prolonged. The cross-linking reaction occurs with the aldehyde group on glutaraldehyde, resulting in a significant reduction in the number of hydroxyl groups in the HPMC molecule, thus prolonging the water solubility of the HPMC film and reducing the water solubility of the HPMC film.
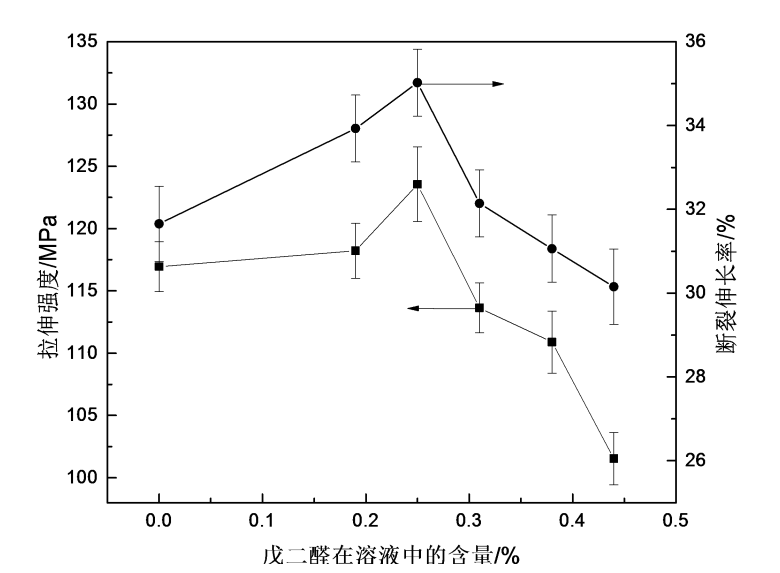
4.3.4 Effect of glutaraldehyde on mechanical properties of HPMC films
Fig.4.4 The effect of glutaraldehyde on tensile strength and breaking elongation of HPMC films
In order to investigate the effect of glutaraldehyde content on the mechanical properties of HPMC films, the tensile strength and elongation at break of the modified films were tested. For example, 4.4 is the graph of the effect of glutaraldehyde addition on the tensile strength and elongation at break of the film. With the increase of glutaraldehyde addition, the tensile strength and elongation at break of HPMC films increased first and then decreased. the trend of. Since the cross-linking of glutaraldehyde and cellulose belongs to etherification cross-linking, after adding glutaraldehyde to the HPMC film, the two aldehyde groups on the glutaraldehyde molecule and the hydroxyl groups on the HPMC molecule undergo a cross-linking reaction to form ether bonds, increasing the mechanical properties of HPMC films. With the continuous addition of glutaraldehyde, the cross-linking density in the solution increases, which limits the relative sliding between molecules, and the molecular segments are not easily oriented under the action of external force, which shows that the mechanical properties of HPMC thin films decline macroscopically [76] ]. From Figure 4.4, the effect of glutaraldehyde on the mechanical properties of HPMC films shows that when the addition of glutaraldehyde is 0.25%, the crosslinking effect is better, and the mechanical properties of HPMC films are better.
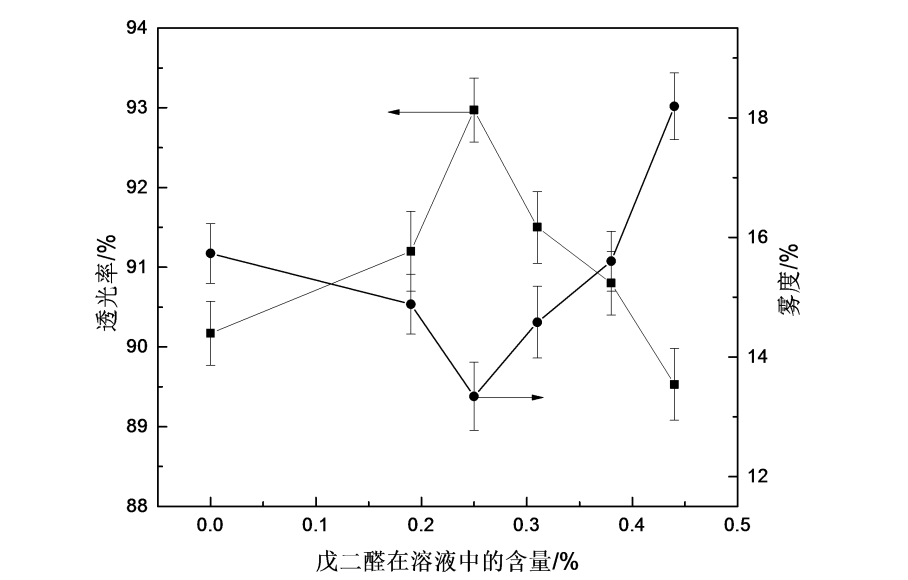
4.3.5 The effect of glutaraldehyde on the optical properties of HPMC films
Light transmittance and haze are two very important optical performance parameters of packaging films. The greater the transmittance, the better the transparency of the film; the haze, also known as turbidity, indicates the degree of indistinctness of the film, and the greater the haze, the worse the clarity of the film. Figure 4.5 is the influence curve of the addition of glutaraldehyde on the optical properties of HPMC films. It can be seen from the figure that with the increase of the addition of glutaraldehyde, the light transmittance first slowly increases, then increases rapidly and then decreases slowly; haze It first decreased and then increased. When the addition of glutaraldehyde was 0.25%, the transmittance of HPMC film reached the maximum value of 93%, and the haze reached the minimum value of 13%. At this time, the optical performance was better. The reason for the increase in optical properties is the cross-linking reaction between glutaraldehyde molecules and hydroxypropyl methylcellulose, and the intermolecular arrangement is more compact and uniform, which increases the optical properties of HPMC films [77-79]. When the cross-linking agent is excessive, the cross-linking sites are supersaturated, the relative sliding between the molecules of the system is difficult, and the gel phenomenon is easy to occur. Therefore, the optical properties of HPMC films are reduced [80].
Fig.4.5 The effect of glutaraldehyde on optical property of HPMC films
4.4 Sections of this chapter
Through the above analysis, the following conclusions are drawn:
1) The infrared spectrum of the glutaraldehyde-crosslinked HPMC film shows that the glutaraldehyde and HPMC film undergo a cross-linking reaction.
2) It is more appropriate to add glutaraldehyde in the range of 0.25% to 0.44%. When the addition amount of glutaraldehyde is 0.25%, the comprehensive mechanical properties and optical properties of the HPMC film are better; after cross-linking, the water solubility of the HPMC film is prolonged and the water solubility is reduced. When the addition amount of glutaraldehyde is 0.44%, the water solubility time Reach about 135min.
Chapter 5 Natural Antioxidant HPMC Water Soluble Packaging Film
5.1 Introduction
In order to expand the application of hydroxypropyl methylcellulose film in food packaging, this chapter uses bamboo leaf antioxidant (AOB) as a natural antioxidant additive, and uses solution casting film-forming method to prepare natural bamboo leaf antioxidants with different mass fractions. Antioxidant HPMC water-soluble packaging film, study the antioxidant properties, water solubility, mechanical properties and optical properties of the film, and provide a basis for its application in food packaging systems.
5.2 Experimental part
5.2.1 Experimental materials and experimental instruments
Tab.5.1 Experimental materials and specifications
Tab.5.2 Experimental apparatus and specifications
5.2.2 Specimen Preparation
Prepare hydroxypropyl methylcellulose water-soluble packaging films with different amounts of bamboo leaf antioxidants by solution casting method: prepare 5% hydroxypropyl methylcellulose aqueous solution, stir evenly, and then add hydroxypropyl methylcellulose Add a certain proportion (0%, 0.01%, 0.03%, 0.05%, 0.07%, 0.09%) of bamboo leaf antioxidants to the cellulose film-forming solution, and continue to stir
To be fully mixed, let stand at room temperature for 3-5 minutes (defoaming) to prepare HPMC film-forming solutions containing different mass fractions of bamboo leaf antioxidants. Dry it in a blast drying oven, and put it in a drying oven for later use after peeling off the film. The prepared hydroxypropyl methylcellulose water-soluble packaging film added with bamboo leaf antioxidant is referred to as AOB/HPMC film for short.
5.2.3 Characterization and performance testing
5.2.3.1 Infrared absorption spectroscopy (FT-IR) analysis
The infrared absorption spectra of HPMC films were measured in ATR mode using a Nicolet 5700 Fourier transform infrared spectrometer produced by Thermoelectric Corporation.
5.2.3.2 Wide-angle X-ray diffraction (XRD) measurement: same as 2.2.3.1
5.2.3.3 Determination of antioxidant properties
In order to measure the antioxidant properties of the prepared HPMC films and AOB/HPMC films, the DPPH free radical scavenging method was used in this experiment to measure the scavenging rate of the films to DPPH free radicals, so as to indirectly measure the oxidation resistance of the films.
Preparation of DPPH solution: Under shading conditions, dissolve 2 mg of DPPH in 40 mL of ethanol solvent, and sonicate for 5 minutes to make the solution uniform. Store in refrigerator (4°C) for later use.
Referring to the experimental method of Zhong Yuansheng [81], with a slight modification, the measurement of A0 value: take 2 mL of DPPH solution into a test tube, then add 1 mL of distilled water to fully shake and mix, and measure the A value (519nm) with a UV spectrophotometer. is A0. Measurement of A value: add 2 mL of DPPH solution to a test tube, then add 1 mL of HPMC thin film solution to mix thoroughly, measure A value with UV spectrophotometer, take water as blank control, and three parallel data for each group. DPPH free radical scavenging rate calculation method refers to the following formula,
In the formula: A is the absorbance of the sample; A0 is the blank control
5.2.3.4 Determination of mechanical properties: same as 2.2.3.2
5.2.3.5 Determination of optical properties
Optical properties are important indicators of the transparency of packaging films, mainly including the transmittance and haze of the film. The transmittance and haze of the films were measured using a transmittance haze tester. The light transmittance and haze of the films were measured at room temperature (25°C and 50% RH) on test samples with clean surfaces and no creases.
5.2.3.6 Determination of water solubility
Cut a 30mm×30mm film with a thickness of about 45μm, add 100mL of water to a 200ml beaker, place the film in the center of the still water surface, and measure the time for the film to disappear completely. If the film sticks to the wall of the beaker, it needs to be measured again, and the result is taken as the average of 3 times, the unit is min.
5.2.4 Data processing
The experimental data were processed by Excel and graphed by Origin software.
5.3 Results and Analysis
5.3.1 FT-IR analysis
Fig5.1 FTIR of HPMC and AOB/HPMC films
In organic molecules, the atoms that form chemical bonds or functional groups are in a state of constant vibration. When the organic molecules are irradiated with infrared light, the chemical bonds or functional groups in the molecules can absorb vibrations, so that information about the chemical bonds or functional groups in the molecule can be obtained. Figure 5.1 shows the FTIR spectra of HPMC film and AOB/HPMC film. From Figure 5, it can be seen that the characteristic skeletal vibration of hydroxypropyl methylcellulose is mainly concentrated in 2600~3700 cm-1 and 750~1700 cm-1. The strong vibration frequency in the 950-1250 cm-1 region is mainly the characteristic region of C-O skeleton stretching vibration. The absorption band of the HPMC film near 3418 cm-1 is caused by the stretching vibration of the O-H bond, and the absorption peak of the hydroxyl group on the hydroxypropoxy group at 1657 cm-1 is caused by the stretching vibration of the framework [82]. The absorption peaks at 1454cm-1, 1373cm-1, 1315cm-1 and 945cm-1 were normalized to Asymmetric, symmetrical deformation vibrations, in-plane and out-of-plane bending vibrations belonging to -CH3 [83]. HPMC was modified with AOB. With the addition of AOB, the position of each characteristic peak of AOB/HPMC did not shift, indicating that the addition of AOB did not destroy the groups of HPMC itself. The stretching vibration of the O-H bond in the absorption band of the AOB/HPMC film near 3418 cm-1 is weakened, and the change of peak shape is mainly caused by the change of the adjacent methyl and methylene bands due to the hydrogen bond induction. 12], it can be seen that the addition of AOB has an effect on intermolecular hydrogen bonds.
5.3.2 XRD analysis
Fig.5.2 XRD of HPMC and AOB/
Fig.5.2 XRD of HPMC and AOB/HPMC films
The crystalline state of the films was analyzed by wide-angle X-ray diffraction. Figure 5.2 shows the XRD patterns of HPMC films and AAOB/HPMC films. It can be seen from the figure that the HPMC film has 2 diffraction peaks (9.5°, 20.4°). With the addition of AOB, the diffraction peaks around 9.5° and 20.4° are significantly weakened, indicating that the molecules of the AOB/HPMC film are arranged in an orderly manner. The ability decreased, indicating that the addition of AOB disrupted the arrangement of hydroxypropyl methylcellulose molecular chain, destroyed the original crystal structure of the molecule, and reduced the regular arrangement of hydroxypropyl methylcellulose.
5.3.3 Antioxidant properties
In order to explore the effect of different AOB additions on the oxidation resistance of AOB/HPMC films, the films with different additions of AOB (0, 0.01%, 0.03%, 0.05%, 0.07%, 0.09%) were investigated, respectively. The effect of the scavenging rate of the base, the results are shown in Figure 5.3.
Fig.5.3 The effect of HPMC films under AOB content on DPPH Inhabition
It can be seen from Figure 5.3 that the addition of AOB antioxidant significantly improved the scavenging rate of DPPH radicals by HPMC films, that is, the antioxidant properties of the films were improved, and with the increase of AOB addition, the scavenging of DPPH radicals first increased then gradually decreased. When the addition amount of AOB is 0.03%, the AOB/HPMC film has the best effect on the scavenging rate of DPPH free radicals, and its scavenging rate for DPPH free radicals reaches 89.34%, that is, the AOB/HPMC film has the best anti-oxidation performance at this time; When the AOB content was 0.05% and 0.07%, the DPPH free radical scavenging rate of the AOB/HPMC film was higher than that of the 0.01% group, but significantly lower than that of the 0.03% group; this may be due to excessive natural antioxidants The addition of AOB led to the agglomeration of AOB molecules and uneven distribution in the film, thus affecting the effect of the antioxidant effect of AOB/HPMC films. It can be seen that the AOB/HPMC film prepared in the experiment has good anti-oxidation performance. When the addition amount is 0.03%, the anti-oxidation performance of the AOB/HPMC film is the strongest.
5.3.4 Water solubility
From Figure 5.4, the effect of bamboo leaf antioxidants on the water solubility of hydroxypropyl methylcellulose films, it can be seen that different AOB additions have a significant effect on the water solubility of HPMC films. After adding AOB, with the increase of the amount of AOB, the water-soluble time of the film was shorter, indicating that the water-solubility of the AOB/HPMC film was better. That is to say, the addition of AOB improves the AOB/HPMC Water solubility of the film. From the previous XRD analysis, it can be seen that after adding AOB, the crystallinity of the AOB/HPMC film is reduced, and the force between the molecular chains is weakened, which makes it easier for water molecules to enter the AOB/HPMC film, so the AOB/HPMC film is improved to a certain extent. Water solubility of the film.
Fig.5.4 The effect of AOB on Water soluble of HPMC films
5.3.5 Mechanical properties
Fig.5.5 The effect of AOB on tensile strength and breaking elongation of HPMC films
The application of thin film materials is more and more extensive, and its mechanical properties have a great influence on the service behavior of membrane-based systems, which has become a major research hotspot. Figure 5.5 shows the tensile strength and elongation at break curves of AOB/HPMC films. It can be seen from the figure that different AOB additions have significant effects on the mechanical properties of the films. After adding AOB, with the increase of AOB addition, AOB/HPMC. The tensile strength of the film showed a downward trend, while the elongation at break showed a trend of first increasing and then decreasing. When the AOB content was 0.01%, the elongation at break of the film reached a maximum value of about 45%. The effect of AOB on the mechanical properties of HPMC films is obvious. From the XRD analysis, it can be seen that the addition of antioxidant AOB reduces the crystallinity of the AOB/HPMC film, thereby reducing the tensile strength of the AOB/HPMC film. The elongation at break first increases and then decreases, because AOB has good water solubility and compatibility, and is a small molecular substance. During the process of compatibility with HPMC, the interaction force between molecules is weakened and the film is softened. The rigid structure makes the AOB/HPMC film soft and the elongation at break of the film increases; as the AOB continues to increase, the elongation at break of the AOB/HPMC film decreases, because the AOB molecules in the AOB/HPMC film make the macromolecules The gap between the chains increases, and there is no entanglement point between the macromolecules, and the film is easy to break when the film is stressed, so that the elongation at break of the AOB/HPMC film decreases.
5.3.6 Optical properties
Fig.5.6 The effect of AOB on optical property of HPMC films
Figure 5.6 is a graph showing the change in transmittance and haze of AOB/HPMC films. It can be seen from the figure that with the increase of the amount of AOB added, the transmittance of the AOB/HPMC film decreases and the haze increases. When the AOB content did not exceed 0.05%, the change rates of light transmittance and haze of AOB/HPMC films were slow; when the AOB content exceeded 0.05%, the change rates of light transmittance and haze were accelerated. Therefore, the amount of AOB added should not exceed 0.05%.
5.4 Sections of this chapter
Taking bamboo leaf antioxidant (AOB) as natural antioxidant and hydroxypropyl methylcellulose (HPMC) as film-forming matrix, a new type of natural antioxidant packaging film was prepared by solution blending and casting film-forming method. The AOB/HPMC water-soluble packaging film prepared in this experiment has the functional properties of anti-oxidation. The AOB/HPMC film with 0.03% AOB has a scavenging rate of about 89% for DPPH free radicals, and the scavenging efficiency is the best, which is better than that without AOB. The HPMC film at 61% improved. The water solubility is also significantly improved, and the mechanical properties and optical properties are decreased. The improved oxidation resistance of AOB/HPMC film materials has expanded its application in food packaging.
Chapter VI Conclusion
1) With the increase of the HPMC film-forming solution concentration, the mechanical properties of the film first increased and then decreased. When the HPMC film-forming solution concentration was 5%, the mechanical properties of the HPMC film were better, and the tensile strength was 116MPa. The elongation at break is about 31%; the optical properties and water solubility decrease.
2) With the increase of the film forming temperature, the mechanical properties of the films first increased and then decreased, the optical properties improved, and the water solubility decreased. When the film-forming temperature is 50°C, the overall performance is better, the tensile strength is about 116MPa, the light transmittance is about 90%, and the water-dissolving time is about 55min, so the film-forming temperature is more suitable at 50°C.
3) Using plasticizers to improve the toughness of HPMC films, with the addition of glycerol, the elongation at break of HPMC films increased significantly, while the tensile strength decreased. When the amount of glycerol added was between 0.15% and 0.25%, the elongation at break of the HPMC film was about 50%, and the tensile strength was about 60MPa.
4) With the addition of sorbitol, the elongation at break of the film increases first and then decreases. When the addition of sorbitol is about 0.15%, the elongation at break reaches 45% and the tensile strength is about 55MPa.
5) The addition of two plasticizers, glycerol and sorbitol, both decreased the optical properties and water solubility of HPMC films, and the decrease was not great. Comparing the plasticizing effect of the two plasticizers on HPMC films, it can be seen that the plasticizing effect of glycerol is better than that of sorbitol.
6) Through infrared absorption spectroscopy (FTIR) and wide-angle X-ray diffraction analysis, the cross-linking of glutaraldehyde and HPMC and the crystallinity after cross-linking were studied. With the addition of the cross-linking agent glutaraldehyde, the tensile strength and elongation at break of the prepared HPMC films first increased and then decreased. When the addition of glutaraldehyde is 0.25%, the comprehensive mechanical properties of HPMC films are better; after cross-linking, the water-solubility time is prolonged, and the water-solubility decreases. When the addition of glutaraldehyde is 0.44%, the water-solubility time reaches about 135min.
7) Adding an appropriate amount of AOB natural antioxidant to the film-forming solution of HPMC film, the prepared AOB/HPMC water-soluble packaging film has the functional properties of anti-oxidation. The AOB/HPMC film with 0.03% AOB added 0.03% AOB to scavenge DPPH free radicals The removal rate is about 89%, and the removal efficiency is the best, which is 61% higher than that of the HPMC film without AOB. The water solubility is also significantly improved, and the mechanical properties and optical properties are decreased. When the addition amount of 0.03% AOB, the anti-oxidation effect of the film is good, and the improvement of the anti-oxidation performance of AOB/HPMC film expands the application of this packaging film material in food packaging.
Post time: Sep-29-2022